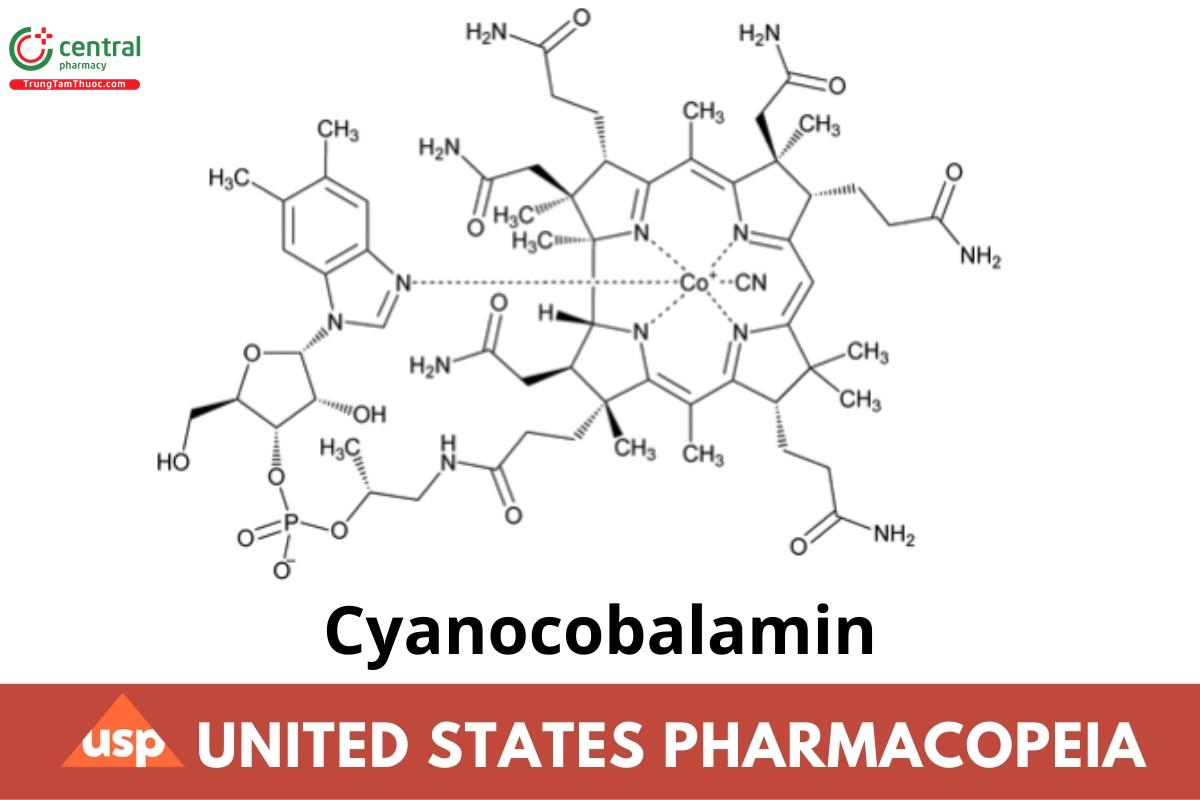
Cyanocobalamin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Cyanocobalamin contains NLT 96.0% and NMT 102.0% of cyanocobalamin (C63H88CoN14O14P), calculated on the dried basis.
2 IDENTIFICATION
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 197U
Wavelength range: 200-700 nm
Sample solution: Prepare as directed in the Assay.
Acceptance criteria: The absorption spectrum exhibits maxima at 278 ± 1, 361 ± 1, and 550 ± 2 nm. The absorbance ratio A361/A278 Is 1.70-1.90, and the absorbance ratio A361/A278 is 3.15-3.40.
2.2 B.
Sample solution: Fuse 1 mg of Cyanocobalamin with 50 mg of potassium pyrosulfate in a porcelain crucible. Cool, break up the mass with a glass rod, add 3 mL of water, and dissolve by boiling.
Analysis: Add 1 drop of phenolphthalein TS, and add sodium hydroxide solution (100 mg/mL), dropwise, until just pink. Add 500 mg of sodium acetate, 0.5 mL of 1 N acetic acid, and 0.5 mL of nitroso R salt solution (2 mg/mL). Add 0.5 mL of hydrochloric acid, and boil for 1 min.
Acceptance criteria: A red or orange-red color appears immediately after the addition of nitroso R salt. The red color persists after boiling with the addition of hydrochloric acid.
2.3 C. HPLC
Mobile phase and Chromatographic system: Proceed as directed in the test for Related Compounds.
Standard solution: 50 µg/mL of Cyanocobalamin from USP Cyanocobalamin (Crystalline) RS in Mobile phase. Use within 1 h.
Sample solution: 50 µg/mL of Cyanocobalamin in Mobile phase. Use within 1 h.
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to that of the Standard solution.
3 ASSAY
3.1 PROCEDURE
Sample solution: 30 µg/mL of Cyanocobalamin in water
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: UV
Analytical wavelength: 361 nm
Cell: 1 cm
Blank: Water
Analysis
Sample: Sample solution
Calculate the percentage of cyanocobalamin (C63H88CoN14O14P) in the portion of Cyanocobalamin taken:
Result = Au/(As x Cu)
Au = absorbance of the Sample solution
As = specific absorbance (E1%) of cyanocobalamin at 361 nm (100 mL . g-1 . cm-1), 207
Cu = concentration of Cyanocobalamin in the Sample solution (g/mL)
Acceptance criteria: 96.0%-102.0% on the dried basis
4 IMPURITIES
Change to read:
4.1 RELATED COMPOUNDS
Solution A: 10 g/L of disodium hydrogen phosphate, dodecahydrate (USP 1-Dec-2023) in water
Mobile phase: Mixture of methanol and Solution A (26.5: 73.5). Adjust with phosphoric acid to a pH of 3.5.
System suitability solution: Dissolve 25 mg of Cyanocobalamin in 10 mL of water, warming if necessary. Allow to cool, add 5 mL of a 1.0-g/L solution of tosylchloramide sodium and 0.5 mL of 0.05 M hydrochloric acid, and then dilute with water to 25 mL. Shake and allow to stand for 5 min. Dilute 1.0 mL of this solution with Mobile phase to 10 mL, and inject immediately.
Quantitative limit solution: 1 µg/mL of Cyanocobalamin in Mobile phase. Use within 1 h.
Sample solution: 1 mg/mL of Cyanocobalamin in Mobile phase. Use within 1 h.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 361 nm
Column: 4.6-mm × 25-cm; 5-µm packing L7
Column temperature: 35°
Flow rate : 0.8 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Quantitative limit solution
[NOTE-The System suitability solution should exhibit two major peaks, cyanocobalamin and 78,8ẞ-lactocyanocobalamin. The relative retention times for the two peaks are 1.0 and 1.2, respectively.] FICIAL
Suitability requirements
Resolution : NLT 2.5 between cyanocobalamin and 78,8ẞ-lactocyanocobalamin, System suitability solution
Signal-to-noise ratio: NLT 5.0 for the major peak, Quantitative limit solution
Analysis
Sample: Sample solution
[NOTE-The run time should be at least three times the retention time of the cyanocobalamin peak.]
Identify the impurities listed in Table 1, and measure the peak responses.
Calculate the percentage of individual impurities in the portion of Cyanocobalamin taken:
Result = (rU/rT) x 100
rU = peak response of each impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 1. [NOTE-Disregard any peak less than 0.1%.]
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Cyanocobalamin | 1.0 | _ |
| 78,8ẞ-Lactocyanocobalamin | 1.2 | 1.0 |
| 50-Carboxycyanocobalamin | 1.3 (USP 1-Dec-2023) | 0.5 |
| 32-Carboxycyanocobalamin (USP 1-Dec-2023) | 1.4 (USP 1-Dec-2023) | 2.0 |
| 34-Methylcyanocobalamin (USP 1-Dec-2023) | 1.5 (USP 1-Dec-2023) | 1.0 |
| 8-epi-Cyanocobalamin | 2.4 (USP 1-Dec-2023) | 1.0 |
| Any other unidentified impurity | _ | 0.5 |
| Total impurities | _ | 3.0 |
5 SPECIFIC TESTS
5.1 LOSS ON DRYING (731)
Sample: 25 mg
Analysis: Dry the Sample in a suitable vacuum drying apparatus at 105° and at a pressure of NMT 5 mm of mercury for 2 h.
Acceptance criteria: NMT 12.0%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE:
Preserve in tight, light-resistant containers, and store at controlled room temperature.
6.2 USP REFERENCE STANDARDS (11)
USP Cyanocobalamin (Crystalline) RS


