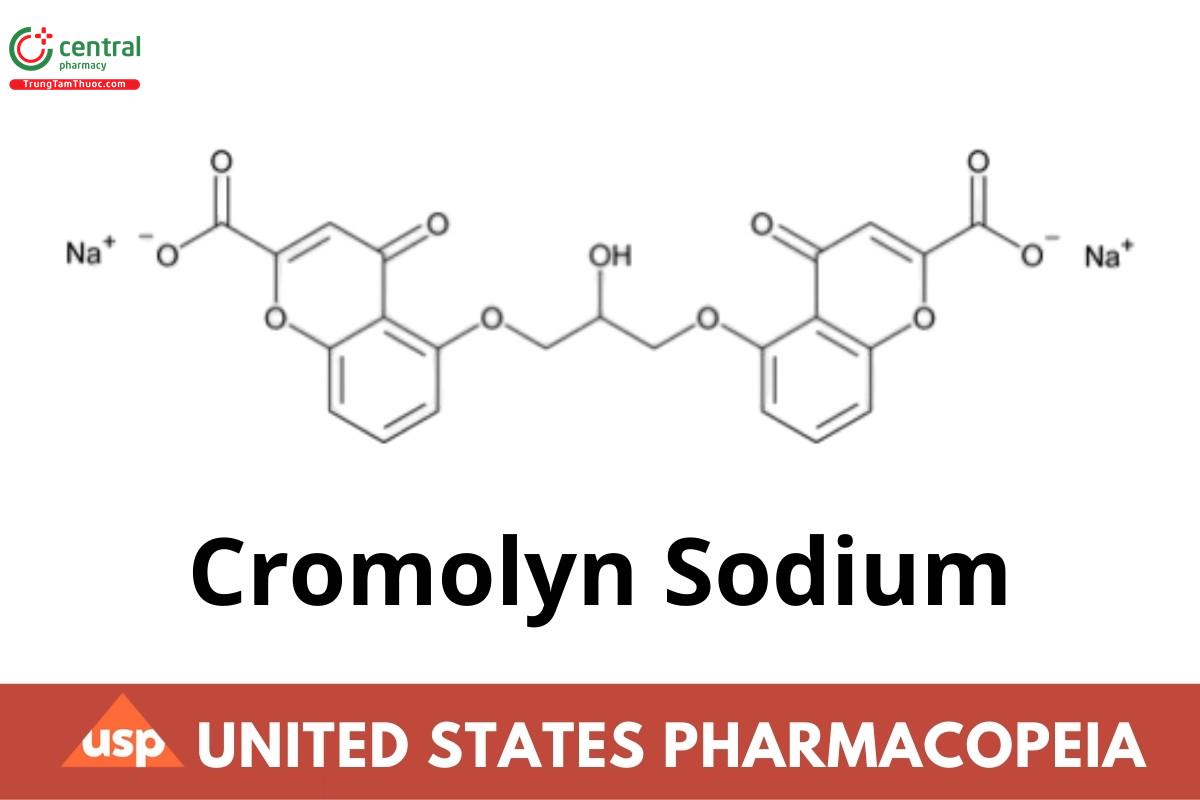
Cromolyn Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
- DEFINITION
- IDENTIFICATION
- A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy:
- B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
- C. IDENTIFICATION TESTS-GENERAL (191), Chemical Identification Tests, Sodium: Meets the requirements
- ASSAY
- IMPURITIES
- SPECIFIC TESTS
- ADDITIONAL REQUIREMENTS
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Cromolyn Sodium contains NLT 98.0% and NMT 101.0% of cromolyn sodium (C23H24Na2O11), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy:
197A or 197K
Analysis: Dry at 105° to constant weight.
Acceptance criteria: Meets the requirements (USP 1-Dec-2023)
2.2 B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
2.3 C. IDENTIFICATION TESTS-GENERAL (191), Chemical Identification Tests, Sodium: Meets the requirements
3 ASSAY
3.1 PROCEDURE
Buffer: 2.1 g/L of sodium acetate in water. Adjust with glacial acetic acid to a pH of 5.5.
Mobile phase: Methanol and Buffer (20:80)
Standard solution: 0.1 mg/mL of USP Cromolyn Sodium RS in water
Sample solution: 0.1 mg/mL of Cromolyn Sodium in water
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Column temperature: 35°
Flow rate: 1.7 mL/min
Injection volume: 20 µL
Run time: NLT 2 times the retention time of cromolyn
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of cromolyn sodium (C23H24Na2O11) in the portion of Cromolyn Sodium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of cromolyn from the Sample solution
rs = peak response of cromolyn from the Standard solution S
Cs = concentration of USP Cromolyn Sodium RS in the Standard solution (mg/mL)
Cu = concentration of Cromolyn Sodium in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-101.0% on the anhydrous basis
4 IMPURITIES
Change to read:
4.1 ORGANIC IMPURITIES
[NOTE-The Sample solution and Standard solutions are stable for 6 h at 4°.]
Diluent A: Acetonitrile and water (20:80)
Diluent B: Methanol and water (30:70)
Buffer: 2.1 g/L of sodium acetate in water. Adjust with glacial acetic acid to a pH of 5.5.
Solution A: Methanol and Buffer (20:80)
Solution B: Methanol
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 5 | 100 | 0 |
| 20 | 43 | 57 |
| 30 | 43 | 57 |
| 32 | 100 | 0 |
| 35 | 100 | 0 |
Standard stock solution: 0.15 mg/mL of USP Cromolyn Related Compound A RS and 0.06 mg/mL of USP Cromolyn Related Compound B RS in acetonitrile
Standard solution A: 0.0075 mg/mL of USP Cromolyn Related Compound A RS and 0.003 mg/mL of USP Cromolyn Related Compound B RS from the Standard stock solution in Diluent A
Standard solution B: 0.0015 mg/mL of USP Cromolyn Sodium RS in Diluent B
Sensitivity solution: 0.75 µg/mL of USP Cromolyn Sodium RS from Standard solution B in Diluent B
Sample solution: 1.5 mg/mL of Cromolyn Sodium in Diluent B
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Temperatures
Autosampler: 4°
Column: 35°
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Samples: Standard solution A, Standard solution B, and Sensitivity solution
[NOTE- The relative retention times in Table 2 are provided as information that could aid in peak assignment.]
Table 2
| Name | Relative Retention Time |
| Cromolyn | 1.0 |
| Cromolyn tricarboxylic acid analog | 1.57 |
| 2-Acetylresorcinol | 2.5 |
| Cromolyn related compound A | 4.2 |
| Cromolyn related compound B | 4.35 (USP 1-Dec-2023) |
a 5-(3-[(2-Carboxy-4-oxo-4H-chromen-5-yl)oxy]-2-hydroxypropoxy)-8-(3-[(2-carboxy-4-oxo-4H-chromen-5-yl)oxy]-2-hydroxypropyl)-4-oxo-4H-chromene-2-carboxylic acid.
b 1-(2,6-Dihydroxyphenyl)ethan-1-one; also known as 2,6-dihydroxyacetophenone.
Suitability requirements
Resolution: NLT 2.5 between cromolyn related compound A and cromolyn related compound B, Standard solution A
Relative standard deviation: NMT 5.0%, Standard solution B
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Calculate the percentage of cromolyn related compound A and cromolyn related compound BA (USP 1-Dec-2023) in the portion of Cromolyn Sodium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of cromolyn related compound A or cromolyn related compound B from the Sample solution
rs = peak response of cromolyn related compound A or cromolyn related compound B from Standard solution A
Cs = concentration of the corresponding cromolyn related compound in Standard solution A (mg/mL)
Cu = concentration of Cromolyn Sodium in the Sample solution (mg/mL)
Calculate the percentage of cromolyn tricarboxylic acid analog. (USP 1-Dec-2023) 2-acetylresorcinol, and any (USP 1-Dec-2023) unspecified impurity in the portion of Cromolyn Sodium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of cromolyn tricarboxylic acid analog, 2-acetylresorcinol, or any (USP 1-Dec-2023) unspecified impurity from the Sample solution
rs = peak response of cromolyn from Standard solution B
Cs = concentration of USP Cromolyn Sodium RS in Standard solution B (mg/mL)
Cu = concentration of Cromolyn Sodium in the Sample solution (mg/mL)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
| Name | Acceptance Criteria, NMT (%) |
| Cromolyn tricarboxylic acid analog | 0.25 |
| 2-Acetylresorcinol | 0.10 |
| Cromolyn related compound A | 0.10 |
| Cromolyn related compound B | 0.10 |
Any (USP 1-Dec-2023) unspecified impurity | 0.10 |
| Total impurities | 0.5 |
4.2 LIMIT OF OXALATE
Standard solution: To 0.35 mg of oxalic acid in 20 mL of water, add 5.0 mL of iron salicylate TS and dilute with water to 50 mL.
Sample solution: To 100 mg of Cromolyn Sodium in 20 mL of water, add 5.0 mL of iron salicylate TS and dilute with water to 50 mL.
Instrumental conditions
Mode: Vis
Analytical wavelength: 480 nm
Blank: Water
Analysis
Samples: Standard solution and Sample solution
Determine the absorbance of the Standard solution and the Sample solution at 480 nm.
Acceptance criteria: The absorbance of the Sample solution is no less than that of the Standard solution (NMT 0.35% of oxalate).
5 SPECIFIC TESTS
5.1 WATER DETERMINATION (921), Method I: NMT 10.0%
5.2 STERILITY TESTS (71): Where the label states that it is sterile, it meets the requirements.
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE:
Preserve in tight containers.
6.2 LABELING:
Where it is intended for use in preparing sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of sterile dosage forms.
6.3 USP REFERENCE STANDARDS (11)
USP Cromolyn Sodium RS
USP Cromolyn Related Compound A RS
1,3-Bis(2-acetyl-3-hydroxyphenoxy)propan-2-ol.
C19H20O7 360.36
USP Cromolyn Related Compound B RS
Diethyl 5,5'-[(2-hydroxypropane-1,3-diyl)bis(oxy)]bis(4-oxo-4H-chromene-2-carboxylate).
C27H24O11 524.48


