Copovidone
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
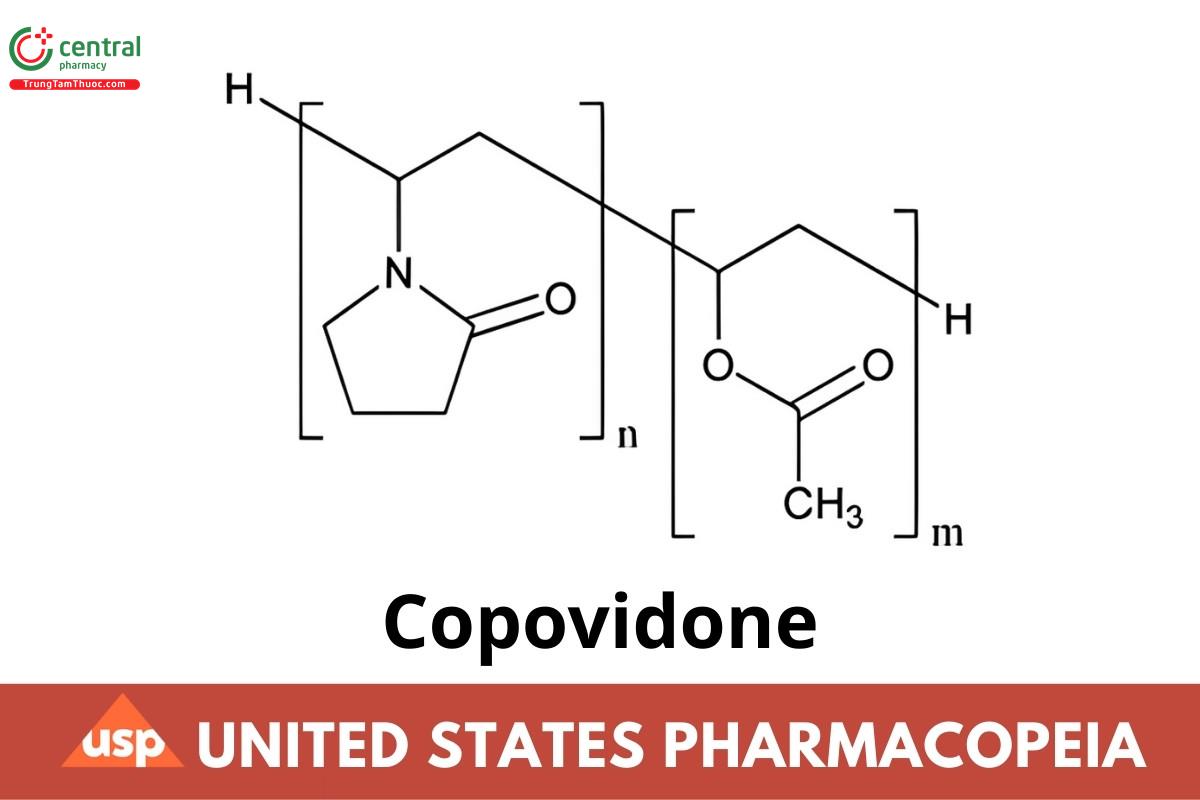
(C6H9NO)n + (C4H6O2)m (n= 1.16m) (NF 1-May-2021)
Acetic acid ethenyl ester polymer with 1-ethenyl-2-pyrrolidone;
1-Vinyl-2-pyrrolidone polymer with vinyl acetate;
(Poly[(2-oxopyrrolidin-1-yl)ethylene-co-(1-acetoxyethylene)]);
Copolymer of 1-ethenylpyrrolidin-2-one and ethenyl acetate (NF 1-May-2021)
CAS RN®: 25086-89-9.
1 DEFINITION
Change to read:
Copovidone is a copolymer of 1-vinyl-2-pyrrolidone and vinyl acetate in the mass proportion of 3:2. It contains NLT 7.0% and NMT 8.0% of nitrogen (N: 14.01), and NLT 35.3% and NMT 42.0% of vinyl acetate (CHO2: 86.09), calculated on the dried basis (NF 1-May-2021).
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
Sample: Dry at 105° for 3 h.
Acceptance criteria: Meets the requirements (NF 1-May-2021)
Change to read:
(NF 1-MAY-2021) B.
Sample solution: 20 mg/mL
Analysis: To 5 mL of the Sample solution add a few drops of iodine TS.
Acceptance criteria: A deep red color is produced (NF 1-May-2021).
3 ASSAY
Change to read:
3.1 PROCEDURE 1: CONTENT OF COPOLYMERIZED VINYL ACETATE
Sample: 2 g of Copovidone (NF 1-May-2021)
Analysis: Determine the saponification value as directed under Fats and Fixed Oils (401), Procedures, Saponification Value.
Calculate the percentage of copolymerized vinyl acetate in the portion of Copovidone taken:
Result = 0.1 x (Mr1 x Mr2) x S
Mr1 = molecular weight of vinyl acetate, 86.09
Mr2 = molecular weight of potassium hydroxide, 56.11
S = saponication value
Acceptance criteria: 35.3%- 42.0% (NF 1-May-2021) of the copolymerized vinyl acetate component, calculated on the dried basis
3.2 PROCEDURE 2: NITROGEN DETERMINATION (461), Method II
Sample: 0.1 g of Copovidone
Analysis: Proceed as directed using the Sample. In the procedure, use 5 g of a powdered mixture of potassium sulfate, cupric sulfate, and titanium dioxide (33:1:1) instead of potassium sulfate and cupric sulfate (10:1); omit the use of hydrogen peroxide; and heat until the solution has a clear, yellow-green color and the sides of the flask are free from carbonaceous material. Then heat for a further 45 min; add 20 mL of water, instead of 70 mL, after the second heating; and use bromocresol green-methyl red TS instead of methyl red-methylene blue TS. Titrate the distillate with 0.05 N sulfuric acid VS until the color of the solution changes from green through pale grayish blue to pale grayish red-purple.
Acceptance criteria: 7.0%-8.0% of Nitrogen on the dried basis
4 IMPURITIES
Change to read:
4.1 RESIDUE ON IGNITION (281)
Sample: 1 g of Copovidone (NF 1-May-2021)
Acceptance criteria: NMT 0.1%
Change to read:
4.2 LIMIT OF ALDEHYDES
Solution A: 17.4 mg/mL of monobasic potassium phosphate, adjusted if necessary, with 1 N potassium hydroxide to a pH of 9.0
Solution B: Transfer a quantity of lyophilized aldehyde dehydrogenase equivalent to 70 units to a glass vial, and dissolve in 10.0 mL of water. [NOTE-This solution is stable for 8 h at 4°.]
Solution C: 40 mg of ẞ-nicotinamide Adenine dinucleotide in 10 mL of Solution A, in a glass vial. [NOTE-This solution is stable for 4 weeks at 4°.] (NF 1-May-2021)
Standard solution: Transfer an equivalent to about 0.140 g of acetaldehyde ammonia trimer trihydrate and dissolve it in water to make exactly 200 mL. (NF 1-May-2021) Transfer 1 mL of this solution to a 100-mL volumetric flask, and dilute with Solution A to volume.
Sample solution: Transfer an equivalent to about 1 g of Copovidone and dissolve it (NF 1-May-2021) in Solution A to exactly 100 mL in a (NF 1-May-2021) volumetric flask. Insert a stopper into the flask, heat at 60° for 1 h, and cool to room temperature.
Analysis: Pipet 0.5 mL each of the Standard solution, Sample solution, and water (used for the blank test) (NF 1-May-2021) into separate 1-cm cells. Add 2.5 mL of Solution A and 0.2 mL of Solution C to each cell. (NF 1-May-2021) Cover the cells to exclude oxygen. (NF 1-May-2021) Mix by inversion, and allow to stand for 2-3 min at 22 ± 2°. Determine the absorbances of the solutions at a wavelength of 340 nm, using water as a reference. (NF 1-May-2021) Add 0.05 mL of Solution B to each cell. Cover the cells to exclude oxygen. Mix by inversion, and allow to stand for 5 min at 22 ± 2°. Determine the absorbances of the solutions at a wavelength of 340 nm, using water as a reference. (NF 1-May-2021)
Calculate the percentage of aldehydes, expressed as acetaldehyde, in the portion of Copovidone taken:
Result = {[(AU2 - AU1) - (AB2 - AB1)]/[(AS2 - AS1) - (AB2 - AB1)]} x (C/W) x 10
AU2 = absorbance of the solution from the Sample solution, after the addition of Solution B
AU1 = absorbance of the solution from the Sample solution, before the addition of Solution B
AB2 = absorbance of the solution from the blank, (NF 1-May-2021) after the addition of Solution B
AB1 = absorbance of the solution from the blank, (NF 1-May-2021) before the addition of Solution B
AS2 = absorbance of the solution from the Standard solution, after the addition of Solution B
AS1 = absorbance of the solution from the Standard solution, before the addition of Solution B
C = concentration of acetaldehyde in the Standard solution (mg/mL), calculated from the weight of the acetaldehyde ammonia trimer trihydrate with a factor of 0.72. [NOTE-The molar mass of acetaldehyde is 44.05 g/mol, and the molar mass of acetaldehyde ammonia trimer trihydrate is 183.26 g/mol. (44.05 x 3)/183.26 = 0.72] (NF 1-May-2021)
W = weight, calculated on the dried basis, of Copovidone taken to prepare the Sample solution (g)
Acceptance criteria: NMT 0.05%(500 ppm) (NF 1-May-2021)
Change to read:
4.3 LIMIT OF HYDRAZINE
Standard solution: 9 µg/mL of salicylaldazine (NF 1-May-2021) in toluene
Sample solution: Transfer the equivalent of 2.5 g of dried Copovidone to a 50-mL centrifuge tube, add 25 mL of water, and mix to dissolve. Add 500 µL of a 50-mg/mL solution of salicylaldehyde in methanol, stir, (NF 1-May-2021) and heat in a water bath at 60° for 15 min. Allow to cool, add 2.0 mL of toluene, insert a stopper in the tube tightly. (NF 1-May-2021) shake vigorously for 2 min, and centrifuge. Use the clear upper toluene layer.
Chromatographic system
(See Chromatography (621), General Procedures, Thin-Layer Chromatography.)
Adsorbent: 0.25-mm layer of dimethylsilanized chromatographic silica gel with a fluorescent indicator (NF 1-May-2021)
Application volume: 10 µL
Developing solvent system: Methanol and water (2:1)
Analytical wavelength: UV 365 nm (NF 1-May-2021)
Analysis
Samples: Standard solution and Sample solution
Allow the spots to dry, and develop the chromatogram in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Locate the spots on the plate by examination under UV light. Salicylaldazine appears as a fluorescent spot having an R, value of about 0.3, (NF 1-May-2021) and the fluorescence of any salicylaldazine spot from the Sample solution is not more intense than that produced by the spot from the Standard solution.
Acceptance criteria: NMT 1 ppm
Change to read:
4.4 LIMIT OF PEROXIDES
Copovidone solution: 40 mg/mL of Copovidone in water calculated on the dried basis
Sample solution: Transfer 25.0 mL of Copovidone solution to a 50-mL beaker, and add 2 mL of titanium trichloride-sulfuric acid TS. Allow to stand for 30 min at room temperature.
Blank solution: Transfer 25.0 mL of Copovidone solution to a 50-mL beaker, and add 2 mL of 13% sulfuric acid.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: UV-Vis
Analytical wavelength: 405 nm
Cell: 1 cm
Blank: Blank solution
Analysis: Determine the absorbance of the Sample solution.
Acceptance criteria: The absorbance is NMT 0.35 [corresponding to NMT 0.04%(400 ppm). (NF 1-May-2021) expressed as hydrogen peroxide].
Delete the following:
4.5 Limit of Monomers (1-Vinyl-2-Pyrrolidone, Vinyl Acetate, and 2-Pyrrolidone)
Solution A: Water, acetonitrile, and methanol (90:5:5)
Solution B: Water, acetonitrile, and methanol (50:45:5)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 2 | 100 | 0 |
| 26 | 80 | 20 |
| 27 | 0 | 100 |
| 36 | 0 | 100 |
| 38 | 100 | 0 |
Standard stock solution: 0.50 mg/mL of 1-vinyl-2-pyrrolidone, 0.50 mg/mL of vinyl acetate, and 3.0 mg/mL of 2-pyrrolidone in methanol
Standard solution: Standard stock solution in Solution A (1 in 2000)
Sample solution: Dissolve 250 mg of Copovidone in 1 mL of methanol, mix ultrasonically, dilute with water to 10 mL. If necessary, filter to remove undissolved particles.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 205 nm and 235 nm
Columns
Guard: 4.0-mm x 2.5-cm; packing L1
Analytical: 4.0-mm × 25-cm; 5-µm packing L1
Column temperature: 30°
Injection volume: 10 µL
Flow rate: 1.0 mL/min
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 2.0 between the 2-pyrrolidone and vinyl acetate peaks, and NLT 2.0 between the vinyl acetate and 1-vinyl-2-pyrrolidone peaks. [NOTE-According to the above operating conditions, the order of elution is 2-pyrrolidone, vinyl acetate, and 1-vinyl-2-pyrrolidone.]
Relative standard deviation: NMT 2.0% for each analyte, on replicate injections
Analysis
Samples: Standard solution and Sample solution
[NOTE-After each injection of the Sample solution wash the polymeric material of Copovidone from the guard column by passing the Mobile phase through the column backwards for 30 min at the same flow rate.]
Calculate the content of 1-vinyl-2-pyrrolidone in the portion of Copovidone taken:
Result = (ATA/ASA) x (CSA/CT) × 100
ATA = 1-vinyl-2-pyrrolidone peak response from the Sample solution
ASA = 1-vinyl-2-pyrrolidone peak response from the Standard solution
CSA = concentration of 1-vinyl-2-pyrrolidone in the Standard solution (mg/mL)
CT = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Calculate the content of vinyl acetate in the portion of Copovidone taken:
Result = (ATB/ASB) x (CSB/CT) x 100
ATB vinyl acetate peak response from the Sample solution
ASB = vinyl acetate peak response from the Standard solution
CSB = concentration of vinyl acetate in the Standard solution (mg/mL)
C = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Calculate the content of 2-pyrrolidone in the portion of Copovidone taken:
Result = (ATC/ASC) x (CSC/CT) x 100
ATC = 2-pyrrolidone peak response from the Sample solution
ASC = 2-pyrrolidone peak response from the Standard solution
CSC = concentration of 2-pyrrolidone in the Standard solution (mg/mL)
CT = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Acceptance criteria: NMT 0.001% of 1-vinyl-2-pyrrolidone, NMT 0.001% of vinyl acetate, and NMT 0.5% of 2-pyrrolidone (NF 1-May-2021)
Change to read:
4.6 LIMIT OF MONOMERS (1-VINYL-2-PYRROLIDONE AND VINYL ACETATE)
Mobile phase: Water and acetonitrile (23:2)
Standard stock solution: 5 µg/mL of 1-vinyl-2-pyrrolidone and 5 µg/mL of vinyl acetate in methanol
Standard solution: 0.25 µg/mL of 1-vinyl-2-pyrrolidone and 0.25 (ERR 1-May-2021) µg/mL of vinyl acetate, respectively, diluted from the
Standard stock solution in Mobile phase
Sample solution: 25 mg/mL of Copovidone in Mobile phase
[NOTE-Store the Sample solution and both Standard solution at a temperature NMT 10°, and use within 8 h.]
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 235 nm for 1-vinyl-2-pyrrolidone; UV 205 nm for vinyl
Columns
Guard: 4.0-mm x 33-mm; 5-µm packing L1
Analytical: 4.0-mm × 25-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1.0 mL/min
Injection volume: 20 µL
Run time: 40 min
System suitability
Sample: Standard solution
[NOTE-The retention times for 1-vinyl-2-pyrrolidone and vinyl acetate are about 17 and 22 min, respectively.]
Suitability requirements
Resolution: NLT 2.0 between 1-vinyl-2-pyrrolidone and vinyl acetate, at the measuring wavelength of 205 nm
Relative standard deviation: NMT 2.0% for each analyte, on 6 replicate injections of the Standard solution
Analysis
Samples: Standard solution and Sample solution
[NOTE-After each test with the Sample solution, wash the polymeric material of Copovidone from the column by passing the Mobile phase through the column backwards for about 30 min at the same Flow rate.]
Calculate the content of 1-vinyl-2-pyrrolidone in the portion of Copovidone taken:
Result = (ATA/ASA) x (CSA/CT) x 100
ATA = peak response of 1-vinyl-2-pyrrolidone from the Sample solution
ASA = peak response of 1-vinyl-2-pyrrolidone from the Standard solution
CSA = concentration of 1-vinyl-2-pyrrolidone in the Standard solution (mg/mL)
CT = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Calculate the content of vinyl acetate in the portion of Copovidone taken:
Result = (ATB/ASB) x (CSB/CT) x 100
ATB = peak response of vinyl acetate from the Sample solution
ASB = peak response of vinyl acetate from the Standard solution
CSB = concentration of vinyl acetate in the Standard solution (mg/mL)
CT = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Acceptance criteria
1-Vinyl-2-pyrrolidone: NMT 0.001% (10 ppm)
Vinyl acetate: NMT 0.001% (10 ppm) (NF 1-May-2021)
Add the following:
4.7 Limit of 2-Pyrrolidone
Mobile phase: Water and methanol (19:1)
Standard solution: 45 µg/mL of 2-pyrrolidone in Mobile phase
Sample solution: Accurately weigh about 1 g of Copovidone, transfer to a 100-mL volumetric flask, add 5 mL of methanol, and dissolve by using ultrasonication. Dilute with water to 100 mL.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 205 nm
Columns
Guard: 4.0-mm x 10-mm; 5-µm packing L1
Analytical: 4.6-mm × 15-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 0.8 mL/min
Injection volume: 20 µL
Run time: 30 min
System suitability
Sample: Standard solution
[NOTE-The retention time for 2-pyrrolidone is about 7 min.]
Suitability requirements
Column efficiency: NLT 5000 theoretical plates for the 2-pyrrolidone peak
Symmetry factor: NMT 1.5 for the 2-pyrrolidone peak
Relative standard deviation: NMT 2.0% for 6 replicate injections of Standard solution
Analysis
Samples: Standard solution and Sample solution
[NOTE-After each test with the Sample solution, wash the polymeric material of Copovidone from the column by passing the Mobile phase through the column backward for about 30 min at the same flow rate.]
Calculate the content of 2-pyrrolidone in the portion of Copovidone taken:
Result = (ATA/ASA) x (CSA/CT) × 100
ATA = peak response of 2-pyrrolidone from the Sample solution
ASA = peak response of 2-pyrrolidone from the Standard solution
CSA = concentration of 2-pyrrolidone in the Standard solution (mg/mL)
CT = concentration of Copovidone in the Sample solution on the dried basis (mg/mL)
Acceptance criteria: NMT 0.5% (NF 1-May-2021)
5 SPECIFIC TESTS
Add the following:
5.1 PH (791)
Sample solution: 100 mg/mL of Copovidone in water
Acceptance criteria: 3.0-7.0 (NF 1-May-2021)
Change to read:
5.2 LOSS ON DRYING (731)
Sample: 0.5 g of Copovidone (NF 1-May-2021)
Analysis: Dry a sample at 105° for 3 h.
Acceptance criteria: NMT 5.0%
5.3 CLARITY AND COLOR OF SOLUTION
Sample: 1.0 g of Copovidone
Analysis: Dissolve the Sample in 10 mL of water.
Acceptance criteria: The solution is clear or slightly opalescent and colorless to pale yellow or pale red.
Change to read:
5.4 K-VALUE
Sample solution: Transfer an amount (NF 1-May-2021) of undried Copovidone, equivalent to 1.00 (NF 1-May-2021) g on the dried basis, and transfer (NF 1-May-2021) to a 100-mL volumetric flask, and dissolve in and dilute with water to volume. Allow to stand for 1 h.
Analysis: Determine the viscosity, using a capillary-tube viscometer (see Viscosity-Capillary Methods (911)), of this solution at 25 ± 0.2°.
Calculate the relative K-value of Copovidone:

c = weight on the dried basis, of the specimen tested in each 100.0 mL of solution (g)
z = viscosity of the Sample solution relative to that of water
Ku = nominal K-value stated on the label
Acceptance criteria: 90.0%-110.0% of the nominal K-value stated on the label of the vial as required by the Labeling section (NF 1-May-2021)
6 ADDITIONAL REQUIREMENTS
Change to read:
6.1 (NF 1-MAY-2021) PACKAGING AND STORAGE
Preserve in tight containers. No storage requirements specified. (NF 1-May-2021)
6.2 LABELING
Label it to indicate its nominal K-value.
6.3 USP REFERENCE STANDARDS (11)
USP Copovidone RS


