Colistimethate Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
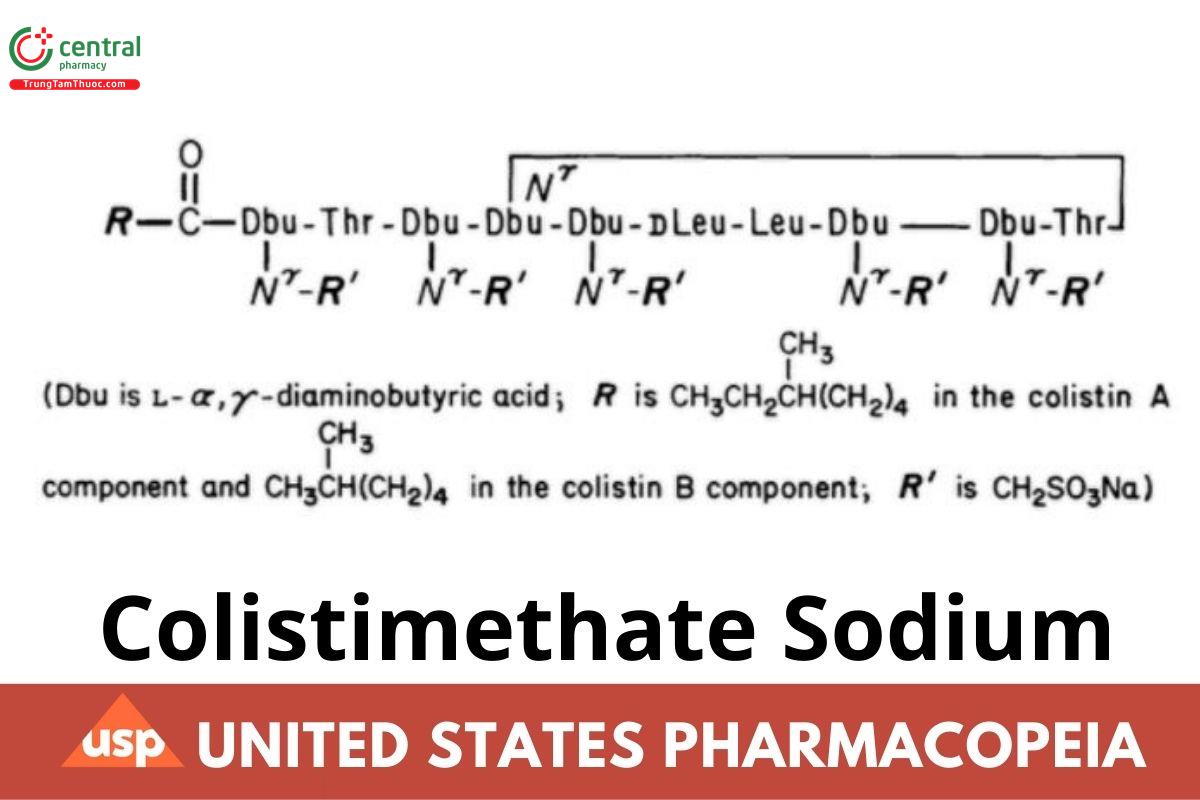
C58H105N16Na5O28S5 (colistin A component) 1749.82
C57H103N16Na5O28S5 (colistin B component) 1735.79
Colistimethate sodium.
Pentasodium colistinmethanesulfonate CAS RN®: 8068-28-8; 21362-08-3 UNII: XW0E5YS77G.
Colistimethate Sodium has a potency equivalent to not less than 390 µg of colistin per mg.
1 Packaging and storage
Preserve as described in Packaging and Storage Requirements 〈659〉, Injection Packaging, Packaging for constitution.
2 Labeling
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
3 USP Reference standards 〈11〉
USP Colistimethate Sodium RS
4 Constituted solution
At the time of use, it meets the requirements for Injections and Implanted Drug Products 〈1〉, Specific Tests, Completeness and clarity of solutions.
Change to read:
5 Identification
5.1 Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
5.2 pH 〈791〉
Between 6.5 and 8.5, in a solution containing 10 mg per mL.
5.3 Loss on drying 〈731〉
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 hours: it loses not more than 7.0% of its weight.
6 Free colistin
Dissolve 80 mg in 3 mL of water, and add 0.05 mL of silicotungstic acid solution (1 in 10): no immediate precipitate is formed.
7 Other requirements
Where the label states that Colistimethate Sodium is sterile, it meets the requirements for Sterility and Bacterial endotoxins under Colistimethate for Injection. Where the label states that Colistimethate Sodium must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Colistimethate for Injection.
8 Assay
Assay preparation-Dissolve a suitable quantity of Colistimethate Sodium, accurately weighed, in 2.0 mL of water, add a sufficient accurately measured volume of Buffer B.6 to obtain a solution having a convenient concentration.
Procedure-Proceed as directed for Colistimethate Sodium under Antibiotics—Microbial Assays 〈81〉, using an accurately measured volume of Assay preparation diluted quantitatively with Buffer B.6 to yield a Test Dilution having a concentration assumed to be equal to the median dose level of the Standard.


