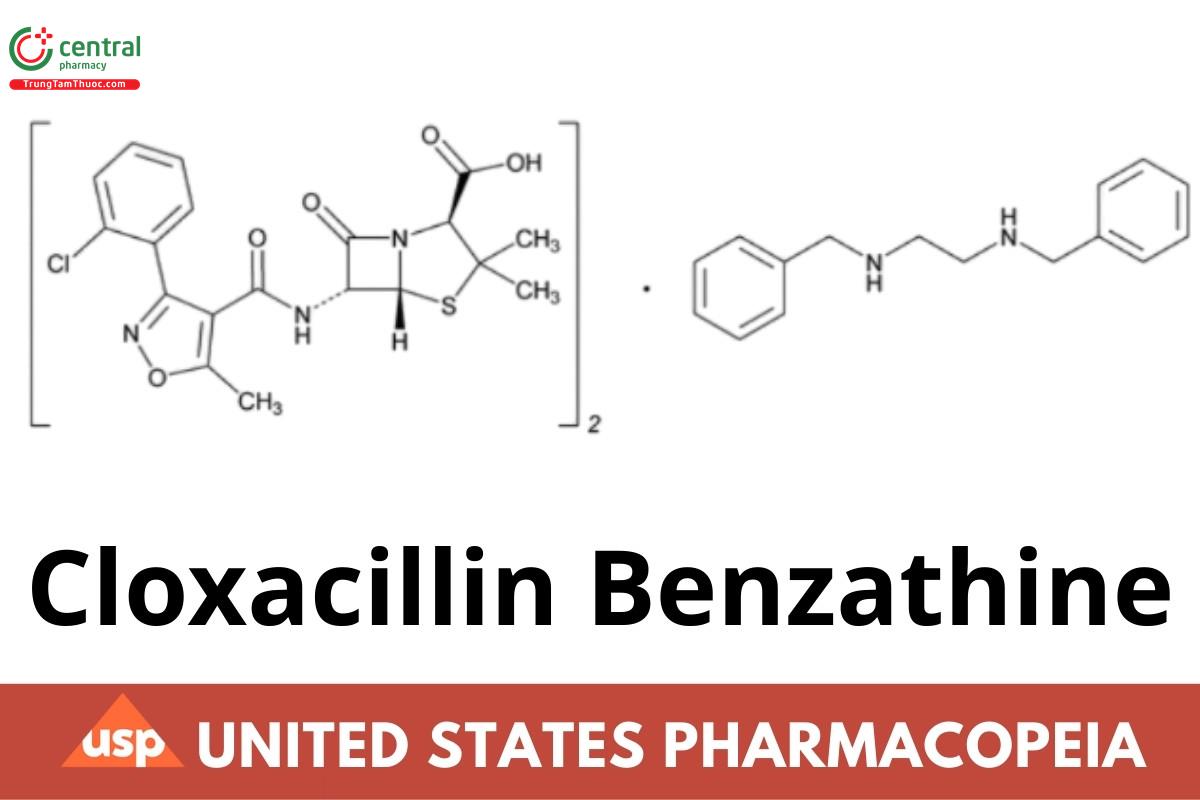
Cloxacillin Benzathine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Cloxacillin Benzathine has a potency equivalent to NLT 704 µg and NMT 821 µg of cloxacillin (C19H18CIN3O5S) per mg, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197)., Infrared Spectroscopy: 197KA (CN 1-MAY-2020)
Change to read:
2.2 B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970 (CN 1-MAY-2020)
Sample solution: To 20 mg of Cloxacillin Benzathine add 5 mL of 5 N sodium hydroxide. Heat the solution on a steam bath for 20 min, and cool. Add 1 mL of this solution to 10 mL of 1.2 N sulfuric acid in a separator, and extract with 50 mL of ether. Wash the ether extract with 30 mL of water, and extract the ether layer with 50 mL of 0.1 N sodium hydroxide.
Standard solution: Use 15 mg of USP Cloxacillin Sodium RS, and follow the same procedures as described in the Sample solution.
Acceptance criteria: Meets the requirements
3 ASSAY
3.1 PROCEDURE
Buffer: 0.1 M monobasic sodium phosphate in water, prepared by dissolving 55.2 g of monobasic sodium phosphate in water, and diluting with water to 4 L
Mobile phase: Acetonitrile and Buffer (1:3). Adjust with phosphoric acid or 1 N sodium hydroxide to a pH of 4.6 ± 0.2. Pass through a 0.45-µm nylon filter, and degas. [NOTE-The retention time of cloxacillin is very sensitive to the acetonitrile content of the Mobile phase.]
Diluent: 0.05 M monobasic sodium phosphate in water. Mix acetonitrile and the resulting solution (2:3). Adjust with phosphoric acid or 1 N sodium hydroxide to a pH of 6.4.
Standard solutions: In duplicate, 112 µg/mL of USP Cloxacillin Sodium RS in Diluent
Sample solutions: In duplicate, 128 µg/mL of Cloxacillin Benzathine in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 10-µm packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
System suitability
Samples: Standard solutions
Suitability requirements: Peak areas of the two Standard solutions agree within 98%-102%.
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2%
Analysis
Samples: Standard solutions and Sample solutions
Calculate the quantity, in µg, of cloxacillin (C19H18CIN3O5S) in each mg of Cloxacillin Benzathine taken:
Result = (ru/rs) × (Cs/Cu) × P
ru = average peak areas of cloxacillin from the Sample solutions
rs = average peak areas of cloxacillin from the Standard solutions s
Cs = concentration of USP Cloxacillin Sodium RS in the Standard solutions (µg/mL)
Cu = concentration of Cloxacillin Benzathine in the Sample solutions (µg/mL)
P = assigned potency of USP Cloxacillin Sodium RS (µg of cloxacillin per mg)
Acceptance criteria: 704-821 µg/mg on the anhydrous basis
4 SPECIFIC TESTS
4.1 CRYSTALLINITY (695):
Meets the requirements
4.2 PH (791).
Sample solution: 10 mg/mL of suspension
Acceptance criteria: 3.0-6.5
4.3 STERILITY TESTS (71):
Where the label states that Cloxacillin Benzathine is sterile, it meets the requirements when tested as directed in Test for Sterility of the Product to Be Examined, Direct Inoculation of the Culture Medium, except use Fluid Thioglycollate Medium containing Polysorbate 80 solution (1 in 200) and an amount of sterile penicillinase sufficient to inactivate the cloxacillin in each tube, use Soybean-Casein Digest Medium containing polysorbate 80 solution (1 in 200) and an amount of sterile penicillinase sufficient to inactivate the cloxacillin in each tube, and shake the tubes once daily.
4.4 WATER DETERMINATION, Method /(921): NMT 5.0%
5 ADDITIONAL REQUIREMENTS
5.1 PACKAGING AND STORAGE: Preserve in tight containers.
5.2 LABELING:
Label it to indicate that it is for veterinary use only. Where it is intended for use in preparing sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of sterile dosage forms.
5.3 USP REFERENCE STANDARDS (11)
USP Cloxacillin Benzathine RS
USP Cloxacillin Sodium RS


