Clomiphene Citrate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
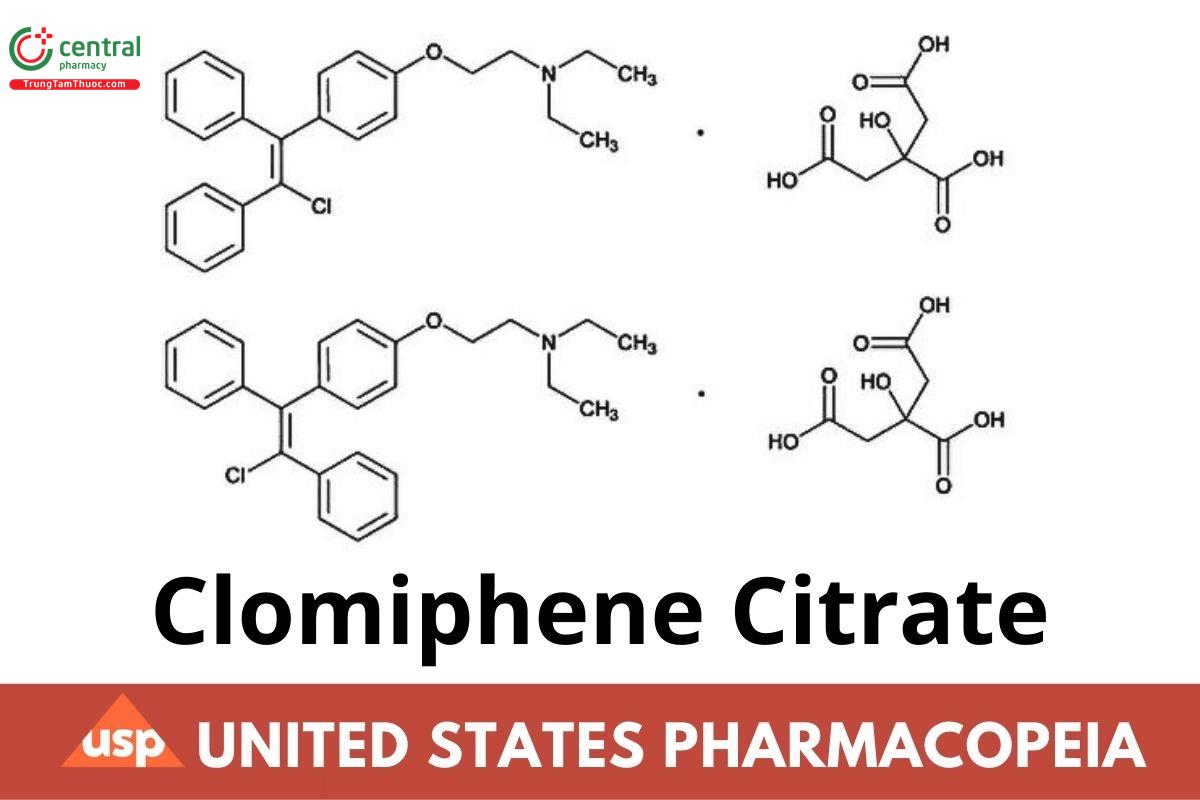
C26H28ClNO.C6H8O7 598.08
Ethanamine, 2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-N,N-diethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1);
2-[p-(2-Chloro-1,2-diphenylvinyl)phenoxy]triethylamine citrate (1:1)
CAS RN®: 50-41-9; UNII: 1B8447E7YI.
1 DEFINITION
Clomiphene Citrate contains NLT 98.0% and NMT 102.0% of a mixture of the (E)- and (Z)-geometric isomers of clomiphene citrate (C26H28ClNO.C6H8O7), calculated on the anhydrous basis. It contains NLT 30.0% and NMT 50.0% of the Z-isomer, [(Z)-2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-N,N-diethylethanamine 2-hydroxy-1,2,3-propanetricarboxylate (1:1)]
2 IDENTIFICATION
2.1 A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
Change to read:
2.2 B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Medium: 0.1 N hydrochloric acid
Sample solution: 20 µg/mL
Acceptance criteria: Meets the requirements
2.3 C. Identification Tests-General, Citrate 〈191〉
3 ASSAY
3.1 Procedure
Use low-actinic glassware for all solutions.
Mobile phase: Methanol, water, and triethylamine (55:45:0.3). Adjust with phosphoric acid to a pH of 2.5.
System suitability solution: 2 µg/mL of USP Clomiphene Related Compound A RS and 50 µg/mL of USP Clomiphene Citrate RS in Mobile phase
Standard solution: 50 µg/mL of USP Clomiphene Citrate RS in Mobile phase
Sample stock solution: 0.5 mg/mL of Clomiphene Citrate in Mobile phase, filtered
Sample solution: 50 µg/mL of Clomiphene Citrate in Mobile phase from Sample stock solution
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 233 nm
- Column: 4.6-mm × 25-cm; packing L26
- Flow rate: 1 mL/min
- Injection volume: 50 µL
System suitability
- Samples: System suitability solution and Standard solution
- [Note-The relative retention times for clomiphene related compound A, (Z)-isomer, and (E)-isomer are about 0.9, 1.0, and 1.2, respectively.]
- Suitability requirements
- Resolution: NLT 1.0 between clomiphene related compound A and (Z)-isomer; NLT 1.5 between (Z)-isomer and (E)-isomer, System suitability solution
- Column efficiency: NLT 2000 theoretical plates for the (E)-isomer, Standard solution
- Tailing factor: NMT 3.0 for the (E)-isomer, Standard solution
- Relative standard deviation: NMT 2.0% for both (E)- and (Z)-isomers, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of clomiphene citrate (C26H28ClNO.C6H8O7) in the portion of Clomiphene Citrate taken:
Result = [(rUE + rUZ)/(rSE + rSZ)] × (CS/CU) × 100
rUE = peak response of the (E)-isomer from the Sample solution
rUZ = peak response of the (Z)-isomer from the Sample solution
rSE = peak response of the (E)-isomer from the Standard solution
rSZ = peak response of the (Z)-isomer from the Standard solution
CS = concentration of USP Clomiphene Citrate RS in the Standard solution (µg/mL)
CU = concentration of Clomiphene Citrate in the Sample solution (µg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
4 OTHER COMPONENTS
4.1 Content of (Z)-isomer
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of (Z)-isomer in the portion of Clomiphene Citrate taken:
Result = (rZ/rT) × 100
rZ = peak response of the (Z)-isomer from the Sample solution
rT = sum of all the peak responses of the Sample solution
Acceptance criteria: 30.0%-50.0%
5 IMPURITIES
5.1 Organic Impurities
Use low-actinic glassware for all solutions.
Buffer: Acetonitrile, diethylamine, and water (40:0.8:60). Adjust with phosphoric acid to a pH of 6.2.
Solution A: Buffer and water (90:10)
Solution B: Buffer
Mobile phase: See Table 1.
| Table 1 | ||
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 3.0 | 100 | 0 |
| 23.0 | 0 | 100 |
| 33.0 | 0 | 100 |
| 33.5 | 100 | 0 |
| 40.0 | 100 | 0 |
System suitability stock solution: 0.28 mg/mL of USP Clomiphene Related Compound A RS in Buffer
System suitability solution: 1.25 mg/mL of USP Clomiphene Citrate RS and 0.028 mg/mL of USP Clomiphene Related Compound A RS, prepared as follows. Transfer 12.5 mg of USP Clomiphene Citrate RS into a 10-mL volumetric flask, add 1.0 mL of System suitability stock solution, and dilute with Buffer to volume.
Standard solution: 0.025 mg/mL of USP Clomiphene Citrate RS in Buffer
Sample solution: 1.25 mg/mL of Clomiphene Citrate in Buffer
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 233 nm
- Column: 4.6-mm × 10-cm; 2.6-µm packing L7
- Column temperature: 30°
- Flow rate: 1.5 mL/min
- Injection volume: 10 µL
System suitability
- Samples: System suitability solution and Standard solution
- [Note-See Table 2 for relative retention times.]
- Suitability requirements
- Relative standard deviation: NMT 5.0% from the sum of the peak areas of the (E)- and (Z)-isomers, Standard solution
- Peak-to-valley ratio: The ratio of the height of the clomiphene related compound A peak to the height of the valley between clomiphene related compound A and clomiphene peaks is NLT 15, System suitability solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Clomiphene Citrate taken:
Result = (ru/rs) × (Cs/Cu) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = sum of the peak responses of (E)- and (Z)-isomers of clomiphene from the Standard solution
Cs = concentration of USP Clomiphene Citrate RS in the Standard solution (mg/mL)
Cu = concentration of Clomiphene Citrate in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. Disregard peaks less than 0.05%.
| Table 2 | |||
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Clomiphene benzophenone analoga | 0.10 | 0.51 | 1.0 |
| Clomiphene tritylphenone analogb | 0.13 | 1.0 | 1.0 |
| Clomiphene keto analogc | 0.33 | 1.0 | 1.0 |
| Clomiphene related compound A | 0.92 | 1.0 | 2.0 |
| Clomiphene Z-isomer | 0.98 | - | - |
| Clomiphene E-isomer | 1.00 | - | - |
| 2-Chloroclomiphened | 1.57 | 1.0 | 1.0e |
| 2-Chloroclomiphened | 1.63 | 1.0 | |
| 4-Chloroclomiphenef | 1.70 | 1.0 | 1.0e |
| 4-Chloroclomiphenef | 1.77 | 1.0 | |
| Deschloroclomiphene chlorophenyl analogg | 2.36 | 0.77 | 1.0e |
| Deschloroclomiphene chlorophenyl analogg | 2.48 | 0.77 | |
| Benzyl clomipheneh | 2.67 | 0.74 | 0.15 |
| Benzyl clomipheneh | 2.76 | 0.81 | 0.15 |
| Any other unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 2.5 |
a {4-[2-(Diethylamino)ethoxy]phenyl}(phenyl)methanone.
b 2,2-Bis{4-[2-(diethylamino)ethoxy]phenyl}-1,2-diphenylethanone.
c 2-{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethan-1-one.
d (E,Z)-2-[2-Chloro-4-(2-chloro-1,2-diphenylvinyl)phenoxy]-N,N-diethylethan-1-amine.
e The sum of these geometric isomers is NMT 1.0%.
f (E,Z)-2-{4-[2-Chloro-2-(4-chlorophenyl)-1-phenylvinyl]phenoxy}-N,N-diethylethan-1-amine.
g (E,Z)-2-{4-[1,2-Bis(4-chlorophenyl)vinyl]phenoxy}-N,N-diethylethan-1-amine.
h (E,Z)-2-{4-[1-(4-Benzylphenyl)-2-chloro-2-phenylvinyl]phenoxy}-N,N-diethylethan-1-amine.
6 SPECIFIC TESTS
Water Determination, Method I 〈921〉: NMT 1.0%
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
- USP Clomiphene Citrate RS
- USP Clomiphene Related Compound A RS
(E,Z)-2-[4-(1,2-Diphenylvinyl)phenoxy]-N,N-diethylethanamine hydrochloride.
Also known as (E,Z)-2-[4-(1,2-Diphenylethenyl)phenoxy]-N,N-diethylethanamine hydrochloride.
C26H29NO·HCl 407.98


