Chlortetracycline Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
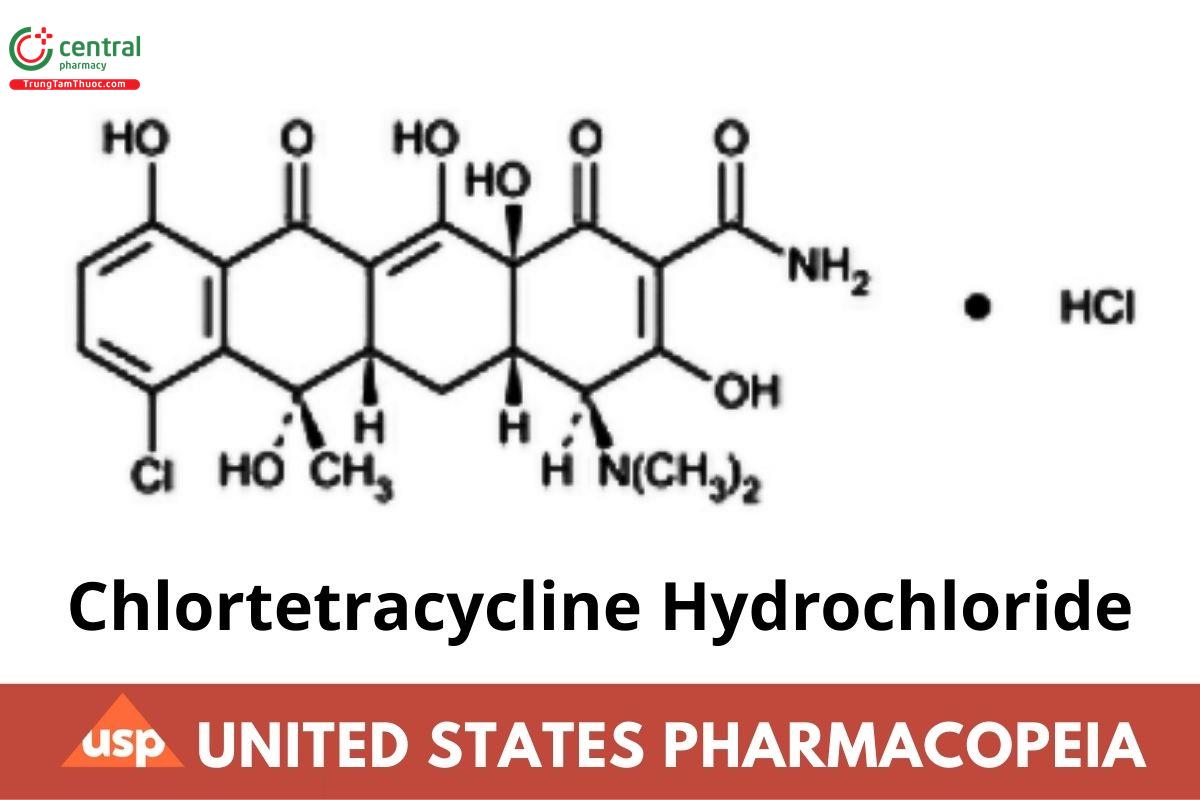
C22H23ClN2O8.HCl 515.34
2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-,
monohydrochloride [4S-(4α,4aα,5aα,6β,12aα)]-;
(4S,4aS,5aS,6S,12aS)-7-Chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2- carboxamide monohydrochloride CAS RN®: 64-72-2; UNII: O1GX33ON8R.
1 DEFINITION
Chlortetracycline Hydrochloride has a potency of NLT 900 µg/mg of chlortetracycline hydrochloride (C22H23ClN2O8.HCl).
[Note—Chlortetracycline Hydrochloride labeled solely for use in preparing oral veterinary dosage forms has a potency of NLT 820 µg/mg of chlortetracycline hydrochloride (C22H23ClN2O8.HCl).]
2 IDENTIFICATION
A. Identification—Tetracyclines 〈193〉, Procedures, Method II
Resolution solution: 0.5 mg/mL each of USP Chlortetracycline Hydrochloride RS, USP Oxytetracycline RS, and USP Tetracycline Hydrochloride RS in methanol
Test solution: 0.5 mg/mL in methanol
Acceptance criteria: Meets the requirements
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride
Sample solution: 1 in 20 solution
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Standard: USP Chlortetracycline Hydrochloride RS
Analysis: Proceed as directed under Antibiotics—Microbial Assays 〈81〉.
Acceptance criteria: NLT 900 µg/mg of chlortetracycline hydrochloride (C22H23ClN2O8.HCl); when labeled solely for use in preparing oral veterinary dosage forms: NLT 820 µg/mg
4 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 5 mg/mL in water that has been allowed to stand in the dark for 30 min
Acceptance criteria: −235° to −250°
Crystallinity 〈695〉: Meets the requirements
pH 〈791〉
Sample solution: 10 mg/mL solution
Acceptance criteria: 2.3–3.3
Loss on Drying 〈731〉
Sample: About 100 mg, accurately weighted
Analysis: Dry Sample in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 h. Acceptance criteria: NMT 2.0%
Sterility Tests 〈71〉
Sample: 3.0% in Fluid D with membrane filtration
Acceptance criteria: Where the label states that Chlortetracycline Hydrochloride is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to Be Examined.
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Labeling: Where it is intended for use in preparing sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of sterile dosage forms.
USP Reference Standards 〈11〉
USP Chlortetracycline Hydrochloride RS
USP Oxytetracycline RS
USP Tetracycline Hydrochloride RS


