Chlorobutanol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
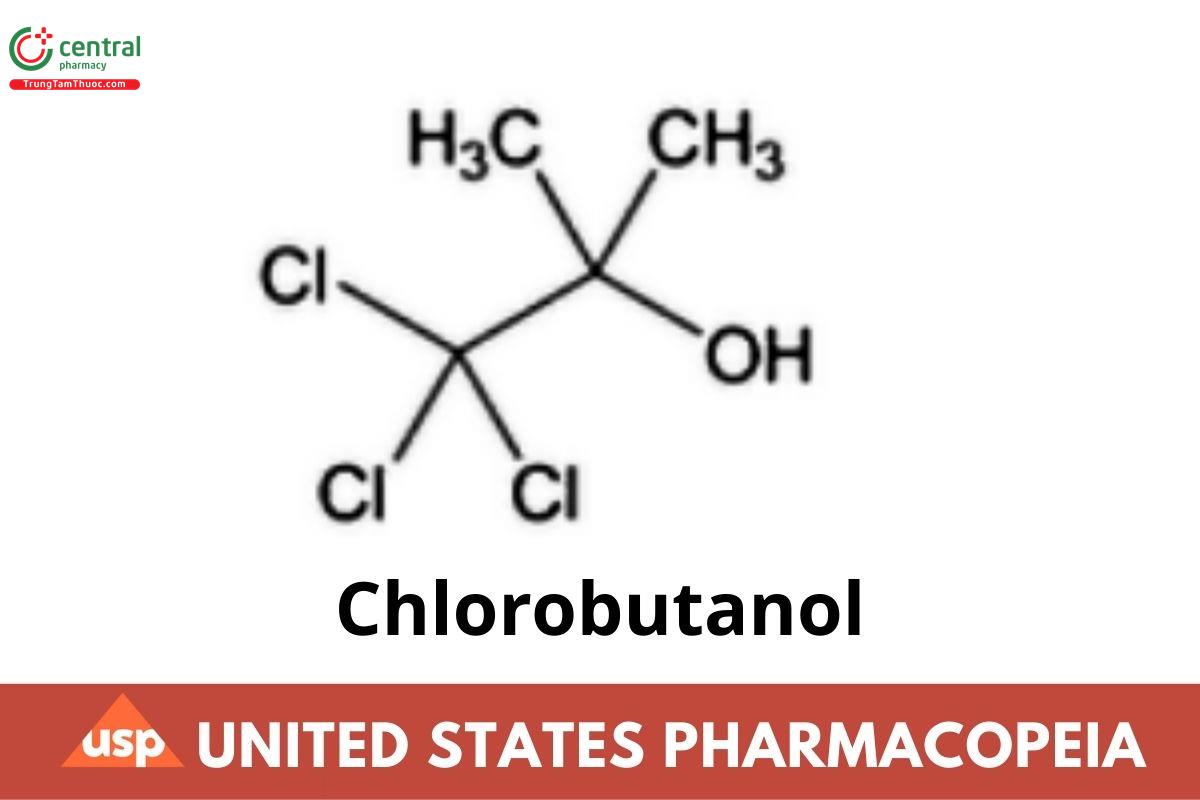
C4H7Cl3O 177.46
C4H7Cl3O · ½H2O 186.46
2-Propanol, 1,1,1-trichloro-2-methyl-;
1,1,1-Trichloro-2-methyl-2-propanol CAS RN®: 57-15-8.
Hemihydrate CAS RN®: 6001-64-5.
1 DEFINITION
Chlorobutanol is anhydrous or contains NMT one-half molecule of water of hydration. It contains NLT 98.0% and NMT 100.5% of chlorobutanol (C4H7Cl3O), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the chlorobutanol peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Standard solution: 10.0 mg/mL of USP Chlorobutanol RS and 15.0 mg/mL of 2,2,2-trichloroethanol (internal standard) in n-hexane Sample solution: 10.0 mg/mL of Chlorobutanol and 15.0 mg/mL of 2,2,2-trichloroethanol (internal standard) in n-hexane
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m fused silica; coated with a 0.25-µm layer of stationary phase G16
Temperatures
Injection port: 260°
Detector: 280°
Column: 135°
Carrier gas: Helium
Flow rate: 1.0 mL/min
Injection volume: 1 µL
Injection type: Split injection, split ratio 10:1
Run time: 12 min
3.2 System suitability
Sample: Standard solution
[Note—The relative retention times for chlorobutanol and 2,2,2-trichloroethanol are 1.0 and 1.3, respectively.] Suitability requirements
Resolution: NLT 5 between the chlorobutanol and 2,2,2-trichloroethanol peaks
Tailing factor: NMT 1.5 for the chlorobutanol peak
Relative standard deviation: NMT 0.3% for peak area ratio of chlorobutanol to the internal standard
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of chlorobutanol (C4H7Cl3O) in the portion of Chlorobutanol taken:
Result = (RU /RS ) × (CS /CU ) × P × 100
RU = peak area ratio of chlorobutanol to the internal standard from the Sample solution
RS = peak area ratio of chlorobutanol to the internal standard from the Standard solution
CS = concentration of USP Chlorobutanol RS in the Standard solution (mg/mL)
CU = concentration of Chlorobutanol in the Sample solution (mg/mL)
P = labeled purity of USP Chlorobutanol RS
Acceptance criteria: 98.0%–100.5% on the anhydrous basis
4 IMPURITIES
4.1 Chloride
Control solution: 0.50 mL of 0.020 N hydrochloric acid in a mixture of 25 mL of diluted alcohol and 1 mL of nitric acid Sample solution: 0.50 g of Chlorobutanol in a mixture of 25 mL of diluted alcohol and 1 mL of nitric acid
Analysis: To the Control solution and Sample solution add 2 mL of silver nitrate TS.
Acceptance criteria: 0.07%; any turbidity produced in the Sample solution is NMT that produced in the Control solution.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 1.0% (anhydrous form) and NMT 6.0% (hydrous form)
Reaction
Sample: 0.5 g
Analysis: Shake the Sample thoroughly with 25 mL of water.
Acceptance criteria: The water remains neutral to litmus.
Bacterial Endotoxins Test 〈85〉: If labeled for use in preparing parenteral dosage forms, it also meets the following requirements. The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Chlorobutanol is used can be met. Where
the label states that Chlorobutanol must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Chlorobutanol is used can be met.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Label it to indicate whether it is anhydrous or hydrous. Where Chlorobutanol is intended for use in the manufacture of injectable dosage forms, it is so labeled. Where Chlorobutanol must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled.
USP Reference Standards 〈11〉
USP Chlorobutanol RS


