Cetrimonium Bromide
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
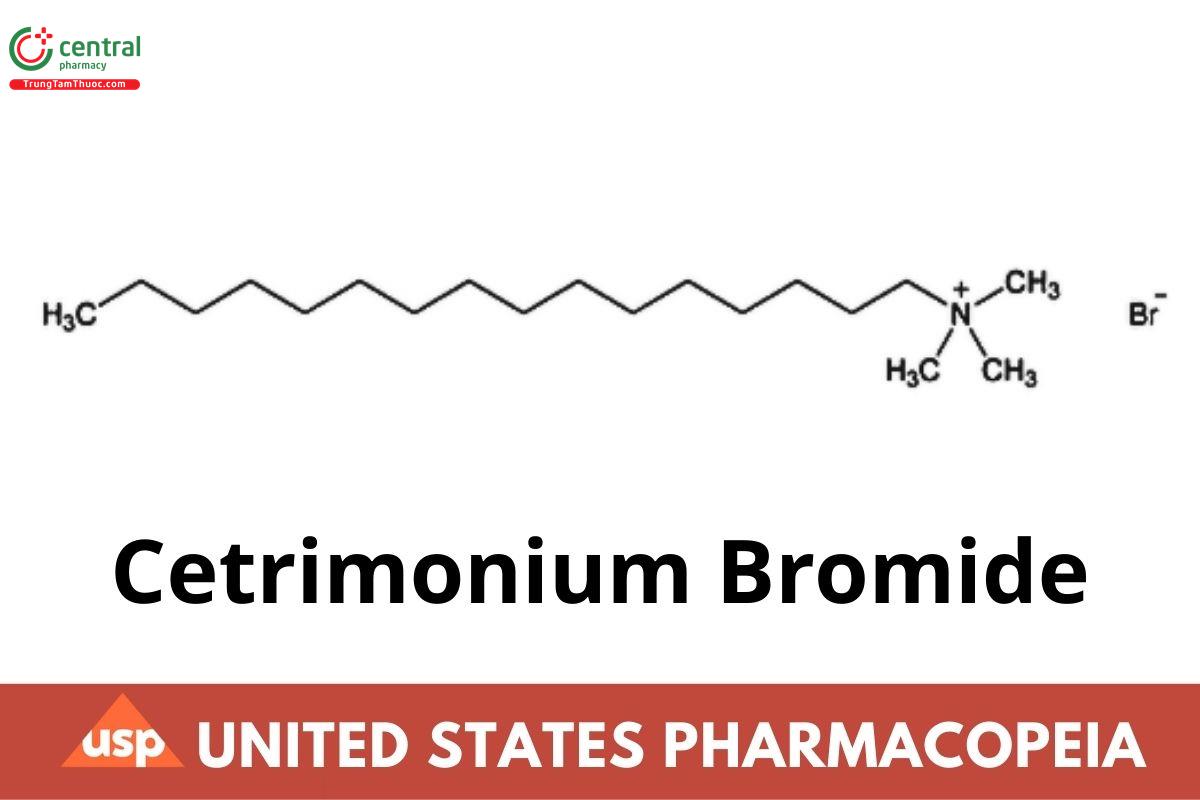
C19H42BrN 364.45
Hexadecyltrimethylammonium bromide CAS RN®: 57-09-0.
1 DEFINITION
Cetrimonium Bromide contains NLT 96.0% and NMT 101.0% of hexadecyltrimethylammonium bromide, calculated as C19H42BrN, on the dried basis.
2 IDENTIFICATION
2.1 A. ULTRAVIOLET ABSORPTION
Sample solution: 10 mg/mL in alcohol
Analytical wavelength: 260-280 nm
Cell: 1 cm
Acceptance criteria: After correcting for the blank, the absorbance is NMT 0.05.
2.2 B.
Sample solution: Dissolve 5 mg of Cetrimonium Bromide in 5 mL of phosphate buffer pH 8.0.
Blank: 5 mL of phosphate buffer pH 8.0
Analysis: Add a strip of methyl green-iodomercurate paper to the Sample solution and Blank.
Acceptance criteria: After 5 min, the Sample solution shows a more intense greenish-blue color than the Blank.
2.3 C.
Sample: 2.0 g
Analysis: Transfer the Sample to a 100-mL flask, and dissolve in and dilute with previously boiled and cooled water to volume.
Acceptance criteria: The solution froths copiously when shaken.
2.4 D. THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST (201)
Solution A: 270 mg/mL of sodium acetate trihydrate
Standard solution: 20 mg/mL of USP Cetrimonium Bromide RS
Sample solution: 20 mg/mL of Cetrimonium Bromide
Chromatographic system
Developing solvent system: Methanol, acetone, and Solution A (45:20:35)
Analysis
Samples: Standard solution and Sample solution
Proceed as directed in the chapter. Remove the plate from the developing chamber, and dry the plate in a current of hot air. Allow to cool.
Expose the plate to iodine vapor, and examine in daylight.
Acceptance criteria: The principal spot of the Sample solution is similar in position, color, and size to that of the Standard solution.
2.5 E. IDENTIFICATION TESTS-GENERAL, Bromide (191): A solution of it meets the requirements.
3 ASSAY
3.1 PROCEDURE
Sample solution: 20 mg/mL of Cetrimorium Bromide
Blank: Combine 10.0 mL of freshly prepared 50-mg/mL potassium iodide, 20 mL of water, and 40 mL of hydrochloric acid.
Titrimetric system
(See Titrimetry (541).)
Mode: Residual titration
Titrant: 0.05 M potassium iodate VS
Analysis
Samples: Sample solution and Blank
Transfer 25.0 mL of the Sample solution to a separatory funnel, and add 25 mL of chloroform, 10 mL of 0.1 N sodium hydroxide VS, and 10.0 mL of a freshly prepared solution of potassium iodide (50 mg/mL). Shake well, allow to separate, and discard the chloroform layer. Wash the aqueous layer with three 10-mL portions of chloroform, and discard the washings. Transfer the aqueous layer to a stoppered conical flask, add 40 mL of hydrochloric acid, and allow to cool. Titrate with Titrant until the deep brown color is almost discharged. Add 2 mL of chloroform, and continue the titration, shaking vigorously, until the color of the chloroform layer no longer changes. Perform a blank determination. Each mL of Titrant is equivalent to 36.45 mg of Cetrimonium Bromide (C19H42BrN).
Acceptance criteria: 96.0%-101.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
Sample: 1.0 g
Acceptance criteria: NMT 0.5%
4.2 LIMIT OF AMINES AND AMINE SALTS
Sample: 5.0 g
Analysis: Dissolve the Sample in 30 mL of a mixture of methanol and 1 N hydrochloric acid VS (99:1), and add 100 mL of isopropyl alcohol. Pass a stream of nitrogen slowly through the solution. Gradually add 15.0 mL of 0.1 N tetrabutylammonium hydroxide VS, recording the potentiometric titration curve.
Acceptance criteria: If the curve shows two inflection points, the volume of titrant added between the two points is NMT 2.0 mL.
5 SPECIFIC TESTS
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62): The total aerobic microbial count is NMT 103 cfu/g. The total
combined molds and yeasts count is NMT 101 cfu/g.
APPEARANCE OF SOLUTION
Analysis: Transfer 2.0 g of Cetrimonium Bromide to a 100-mL flask, and dissolve in and dilute with previously boiled and cooled water to volume.
Acceptance criteria: The solution is clear and colorless.
ACIDITY OR ALKALINITY
Sample solution: 50 mL of the solution obtained in the Appearance of Solution test
Analysis: To the Sample solution add 0.1 mL of bromocresol purple TS.
Acceptance criteria: NMT 0.1 mL of 0.1 N sodium hydroxide or 0.1 N hydrochloric acid is required to change the color of the indicator.
LOSS ON DRYING (731)
Sample: 1.0 g
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 2.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. No storage requirements specified.
USP REFERENCE STANDARDS (11)
USP Cetrimonium Bromide RS


