Cephalexin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
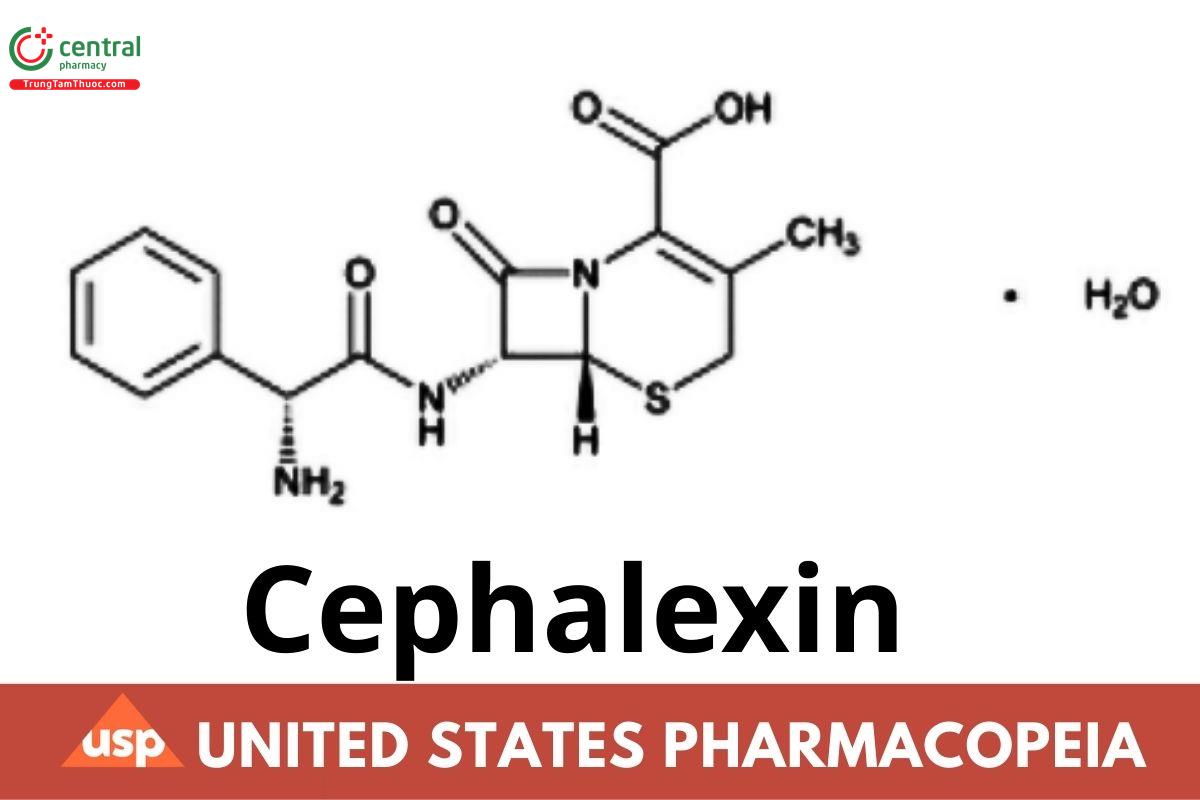
C16H17N3O4S . H2O 365.40
C16H17N3O4S 347.40
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[(aminophenylacetyl)amino]-3-methyl-8-oxo-, monohydrate, [6R-[6α,7β (R*)]]-; (6R,7R)-7-[(R)-2-Amino-2-phenyl acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate CAS RN®: 23325-78-2; UNII: OBN7UDS42Y.
Anhydrous CAS RN®: 15686-71-2; UNII: 5SFF1W6677.
1 DEFINITION
Cephalexin has a potency of NLT 950 µg/mg and NMT 1030 µg/mg of C16H17N3O4S, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: 0.985 g/L of sodium-1-pentanesulfonate in a mixture of acetonitrile, methanol, triethylamine, and water (20:10:3:170), adjusted with phosphoric acid to a pH of 3.0 ± 0.1
Standard stock solution: 1 mg/mL of USP Cephalexin RS in water
Standard solution: 0.4 mg/mL of cephalexin in Mobile phase from Standard stock solution
Sample stock solution: 1 mg/mL of Cephalexin in water
Sample solution: 0.4 mg/mL of Cephalexin in Mobile phase from Sample stock solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; packing L1 of low acidity
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the quantity, in µg, of cephalexin (C16H17N3O4S) per mg of the Cephalexin taken:
Result = (rU/rS) × (CS/CU) × P
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Cephalexin RS in the Standard solution (mg/mL)
CU = concentration of Cephalexin in the Sample solution (mg/mL)
P = potency of USP Cephalexin RS (µg/mg)
Acceptance criteria: 950–1030 µg/mg on the anhydrous basis
4 IMPURITIES
Organic Impurities
Procedure 1
Solution A: Dissolve 1 g of sodium 1-pentanesulfonate in a mixture of 1000 mL of water and 15 mL of triethylamine. Adjust with phosphoric acid to a pH of 2.5 ± 0.1.
Solution B: Dissolve 1 g of sodium 1-pentanesulfonate in a mixture of 300 mL of water and 15 mL of triethylamine. Adjust with phosphoric acid to a pH of 2.5 ± 0.1, and add 350 mL of acetonitrile and 350 mL of methanol.
Mobile phase: See the gradient table below.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 1 | 100 | 0 |
| 33.3 | 0 | 100 |
| 34.3 | 0 | 100 |
Diluent: 18 mg/mL of monobasic potassium phosphate in water
Standard solutions: 0.08 mg/mL and 0.16 mg/mL of cephalexin (C16H17N3O4S) from USP Cephalexin RS in Diluent, taking into account the stated potency of the USP Cephalexin RS
Sample solution: 5 mg/mL of Cephalexin in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; packing L1 of low acidity
Flow rate: 1 mL/min
Injection size: 20 µL
Analysis
Samples: Standard solutions and Sample solution
Plot the responses of the cephalexin peaks from the Standard solutions versus their concentrations, calculated on the anhydrous basis, in mg/mL, and draw a straight line through the two points and zero. From the line and the peak responses of the Sample solution, determine the concentration, I, in mg/mL, of each cephalexin-related substance of the Sample solution other than the cephalexin peak.
Calculate the percentage of each cephalexin-related substance:
Result = I/C × 100
I = concentration of each cephalexin-related substance in the Sample solution as determined from the calibration curve (mg/mL)
C = concentration of cephalexin from the Sample solution (mg/mL)
Acceptance criteria
Individual impurities: NMT 1.0% of any individual cephalexin-related substance
Total impurities: NMT 5.0%
Procedure 2: Dimethylaniline 〈223〉: Meets the requirement
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉: +149° to +158°
Sample solution: 5 mg/mL, in pH 4.4 neutralized phthalate buffer (see Reagents, Indicators, and Solutions—Buffer Solutions) Crystallinity 〈695〉: Meets the requirements
pH 〈791〉: 3.0–5.5, in an aqueous suspension containing 50 mg/mL
Water Determination, Method I〈921〉: 4.0%–8.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Cephalexin RS


