Ceftiofur Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
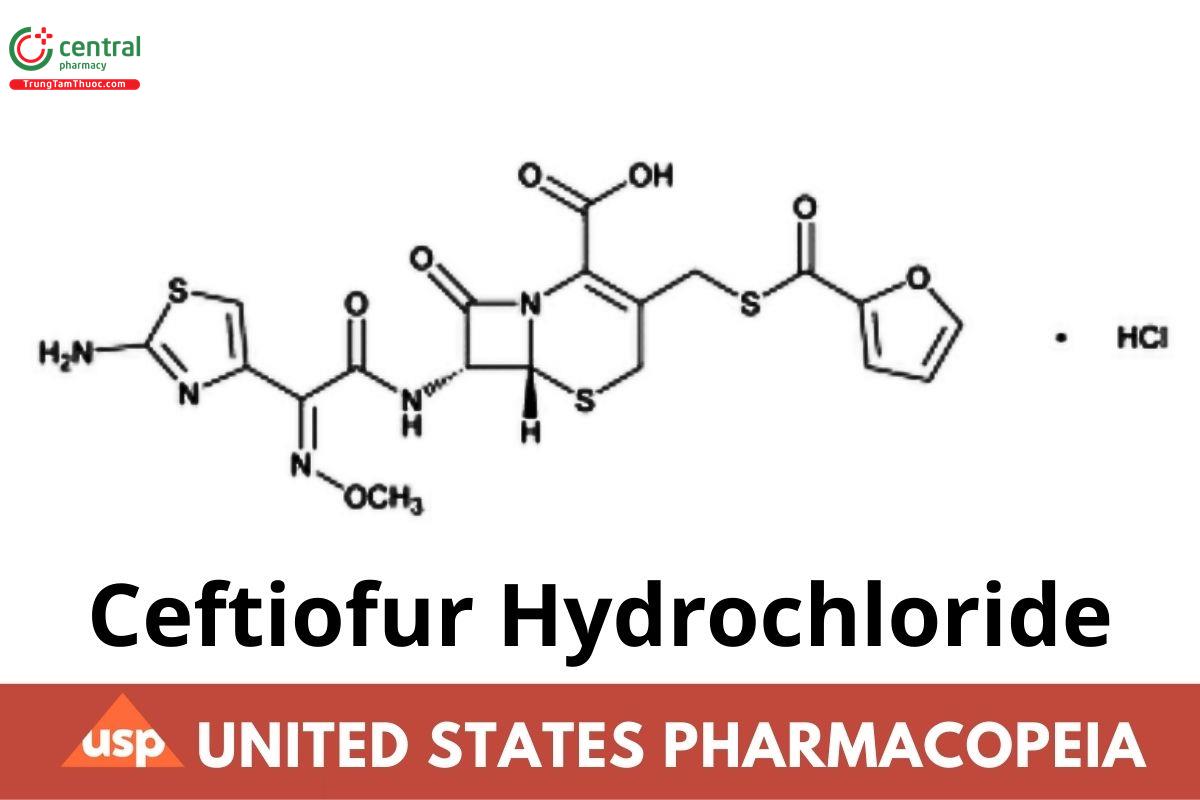
C19H17N5O7S3 .HCl 560.02
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[[(2- furanylcarbonyl)thio]methyl]-8-oxo-, monohydrochloride, [6R-[6α,7β(Z)]]-;
(6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-(O methyloxime), 2-furoate (ester), monohydrochloride CAS RN®: 103980-44-5.
1 DEFINITION
Ceftiofur Hydrochloride contains NLT 844 µg/mg and NMT 956 µg/mg of ceftiofur (C19H17N5O7S3), calculated on the anhydrous and solvent free basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
Procedure
Solution A: Tetrabutylammonium hydroxide, 40% in water
Solution B: Dissolve 3.85 g of ammonium acetate and 13.5 mL of Solution A in 700 mL of water. Adjust with glacial acetic acid to a pH of 6.7. Mobile phase: Mix 700 mL of Solution B with 200 mL of methanol and 110 mL of tetrahydrofuran.
Diluent: 0.05 M ammonium acetate
Standard solution: 0.16 mg/mL of USP Ceftiofur Hydrochloride RS prepared as follows. Dissolve USP Ceftiofur Hydrochloride RS in methanol using about 4% of the nal volume and dilute with Diluent to volume.
Sample solution: 0.16 mg/mL of Ceftiofur Hydrochloride prepared as follows. Dissolve Ceftiofur Hydrochloride in methanol using about 4% of the final volume and dilute with Diluent to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L7
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the quantity, in µg/mg, of ceftiofur (CC19H17N5O7S3) in the portion of Ceftiofur Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Ceftiofur Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Ceftiofur Hydrochloride in the Sample solution (mg/mL)
P = potency of ceftiofur in USP Ceftiofur Hydrochloride RS (µg/mg)
Acceptance criteria: 844–956 µg/mg on the anhydrous and solvent-free basis
4 IMPURITIES
Low Molecular Weight Impurities
Solution A: Acetonitrile, trifluoroacetic acid, and water (50:1:950)
Solution B: Acetonitrile, trifluoroacetic acid, and water (800:1:200)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 5 | 100 | 0 |
| 35 | 60 | 40 |
| 50 | 0 | 100 |
| 55 | 0 | 100 |
| 60 | 100 | 0 |
| 75 | 100 | 0 |
Diluent: Acetonitrile and water (1:1)
System suitability solution: 0.1 mg/mL of USP Ceftiofur System Suitability Mixture RS in Diluent. Sonicate as needed to dissolve. Inject within 20 min of preparation.
Peak identification solution: 15 µg/mL of USP Cefotaxime Sodium RS in Diluent
Sample stock solution: 3 mg/mL of Ceftiofur Hydrochloride in Diluent
Sample solution: 0.3 mg/mL of Ceftiofur Hydrochloride from Sample stock solution in water. Inject the Sample solution within 20 min of preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 3-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: System suitability solution
[Note—The relative retention times for ceftiofur delta-3 isomer and ceftiofur are 0.98 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.5 between ceftiofur delta-3 isomer and ceftiofur
Analysis
Samples: Sample solution and Peak identification solution
[Note—The elution order of the N-deacyl ceftiofur and cefotaxime peaks may be reversed depending on the column used. Determine the location of the cefotaxime peak by using the Peak identification solution.]
Calculate the percentage of each impurity in the portion of Ceftiofur Hydrochloride taken:
Result = {rU/[rS+ Σ(rU/F)]} × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of ceftiofur from the Sample solution
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is 0.05% of the total corrected peak area.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Aminothiazolyl oxime ethyl estera | 0.3 | 1.6 | 0.5 |
| 7-Aminocephal osporanic acidb | 0.10 | 0.77 | 0.5 |
| 2-Furoic acidc | 0.33 | 2.5 | 0.5 |
| N-Deacyl ceftiofurd | 0.7 | 0.61 | 0.5 |
| Cefotaximee | 0.71 | 1.0 | 0.5 |
| Ceftiofur delta-3 isomerf | 0.98 | 1.0 | 0.5 |
| Ceftiofur | 1.0 | - | - |
| Ceftiofur E-isomerg | 1.08 | 1.0 | 3.6 |
| Dihydrothiophenyl thioesterh | 1.2 | 1.0 | 0.5 |
| Ceftiofur amide dimeri | 1.3 | 1.0 | 0.8 |
| N-Trityl ceftiofur oximej | 1.5 | 0.48 | 0.5 |
| Any other individual impurity | - | 1.0 | 0.5 |
| Total impurities | - | - | 6.0 |
a(Z)-Ethyl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetate.
b(6R,7R)-3-(Acetoxymethyl)-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-ACA.
c Furan-2-carboxylic acid.
d(6R,7R)-7-Amino-3-((furan-2-carbonylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
e(6R,7R)-3-(Acetoxymethyl)-7-((Z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
f (6R,7R)-7-((Z)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((furan-2-carbonylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3- ene-2-carboxylic acid.
g(6R,7R)-7-((E)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((furan-2-carbonylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2- ene-2-carboxylic acid.
h S-(4-Hydroxy-5-oxo-2,5-dihydrothiophen-3-yl)methyl furan-2-carbothioate.
i(6R,7S)-7-{(6R,7R)-7-[(Z)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido]-3-[(furan-2-carbonylthio)methyl]-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-enecarboxamido}-3-[(furan-2-carbonylthio)methyl]-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
j (Z)-2-(Methoxyimino)-2-(2-(tritylamino)thiazol-4-yl)acetic acid.
High Molecular Weight Impurities (Ceftiofur Polymers)
To minimize leaching of plastic components, avoid contact between the Mobile phase and plastics during all steps including during the preparation of the Mobile phase, the System suitability solution, and the Sample solution, and when filling the injection vial. Solution A: 45% of potassium hydroxide in water
Solution B: 0.68 g/L of monobasic potassium phosphate. Adjust with Solution A to a pH of 7.5.
Mobile phase: 10 g/L of electrophoresis grade sodium dodecyl sulfate in Solution B. Stir or slightly heat to dissolve. Blank: Use the Mobile phase.
System suitability solution: 0.15 mg/mL of USP Ceftiofur System Suitability Mixture RS in Mobile phase. Sonicate if necessary to dissolve. Inject within 20 min of preparation.
Sample solution: 0.15 mg/mL of Ceftiofur Hydrochloride in Mobile phase. Inject within 20 min of preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.0-mm × 25-cm; 5-µm packing L20
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 2 times the retention time of the ceftiofur peak
System suitability
Sample: System suitability solution
[Note—Adjust the chromatographic system so that the retention time of ceftiofur is NLT 2.5 min. The relative retention times of ceftiofur E isomer and ceftiofur are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.0 between ceftiofur and ceftiofur E-isomer
Analysis
Samples: Blank and Sample solution
Calculate the percentage of high molecular weight impurities in the portion of Ceftiofur Hydrochloride taken:
Result = {100 × (rU/F)/[(rU/F) + rC + rA ]} − T
rU = sum of the responses of all peaks that elute prior to ceftiofur from the Sample solution, corrected for the Blank if necessary
F = relative response factor, 0.8
rC = peak response of ceftiofur from the Sample solution
rA = sum of the responses of all peaks that elute after ceftiofur from the Sample solution
T = total impurities from Table 2
Acceptance criteria
Total high molecular weight impurities: NMT 3.9%
5 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 5 mg/mL in dimethylformamide
Acceptance criteria: −115° to −127° on the anhydrous and solvent-free basis
Bacterial Endotoxins Test 〈85〉: Where the label states that Ceftiofur Hydrochloride is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 1.0 USP Endotoxin Unit/mg of ceftiofur hydrochloride. Water Determination 〈921〉, Method I: NMT 6.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store in a freezer.
Labeling: Label it to indicate that it is intended for veterinary use only. Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms. USP Reference Standards 〈11〉
USP Cefotaxime Sodium RS
USP Ceftiofur Hydrochloride RS
USP Ceftiofur System Suitability Mixture RS
This is a mixture of ceftiofur, ceftiofur delta-3 isomer, ceftiofur E-isomer, and other impurities.
Ceftiofur delta-3 isomer
(6R,7R)-7-((Z)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((furan-2-carbonylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2- carboxylic acid.
C19H17N5O7S3 523.56
Ceftiofur E-isomer
(6R,7R)-7-((E)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((furan-2-carbonylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
C19H17N5O7S3 523.56
USP Endotoxin RS


