Cefprozil
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
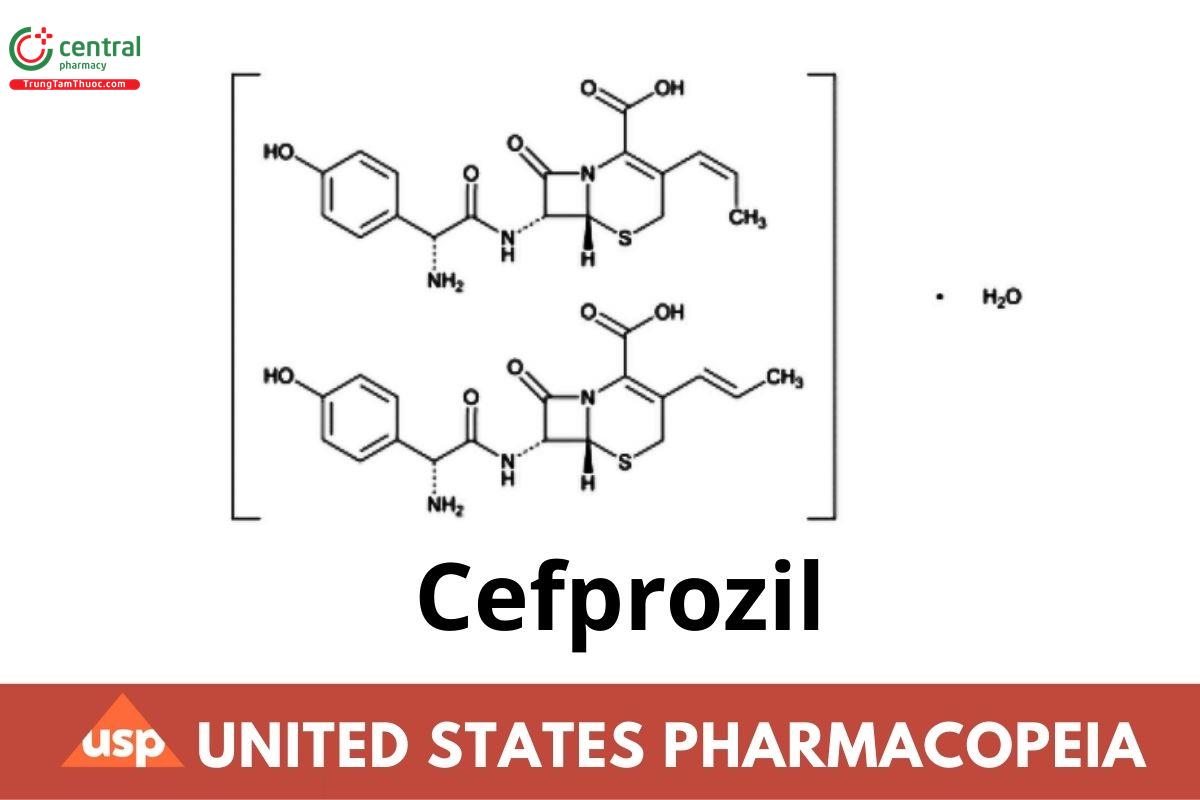
C18H19N3O5S · H O 407.44
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[amino(4-hydroxyphenyl)acetyl]amino]-8-oxo-3-(1-propenyl)-, monohydrate, [6R- [6α,7β(R*)]]-;
(6R,7R)-7-[(R)-2-Amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate CAS RN®: 121123-17-9.
Anhydrous 389.43 CAS RN®: 92665-29-7.
1 DEFINITION
Cefprozil contains NLT 900 µg/mg and NMT 1050 µg/mg of cefprozil (C18H19N3O5S), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K (CN 1-May-2020)
B. The retention times of the cefprozil (Z)-isomer and cefprozil (E)-isomer peaks from the Sample solution correspond to those of the Standard solutions, as obtained in the Assay.
3 ASSAY
Procedure
Buffer: 11.5 g/L of monobasic ammonium phosphate in water. Adjust, if necessary, with phosphoric acid to a pH of 4.4. Mobile phase: Acetonitrile and Buffer (100:900)
System suitability solution: 0.125 mg/mL each of USP Cefprozil (Z)-Isomer RS and USP Cefprozil (E)-Isomer RS in water. Use this solution within 6 h.
Standard solution 1: 0.25 mg/mL of USP Cefprozil (Z)-Isomer RS in water. Use this solution within 6 h.
Standard solution 2: 0.025 mg/mL of USP Cefprozil (E)-Isomer RS in water. Use this solution within 6 h.
Sample solution: 0.3 mg/mL of Cefprozil in water. Shake to dissolve. Use this solution within 6 h.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 30-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution 1
[Note—The relative retention times for cefprozil (Z)-isomer and cefprozil (E)-isomer are about 0.7 and 1.0, respectively.] Suitability requirements
Resolution: NLT 2.5 between cefprozil (Z)-isomer and cefprozil (E)-isomer, System suitability solution
Tailing factor: 0.9–1.1, Standard solution 1
Relative standard deviation: NMT 2.0%, Standard solution 1
Samples: Standard solution 1, Standard solution 2, and Sample solution
Result = (rU/rS) × (CS /CU) × P
rU = peak response of the cefprozil (Z)-isomer from the Sample solution
rS = peak response of the cefprozil (Z)-isomer from Standard solution 1
CS = concentration of USP Cefprozil (Z)-Isomer RS in Standard solution 1 (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of the cefprozil (Z)-Isomer RS (μg/mg)
Calculate the amount (µg/mg) of cefprozil (Z)-isomer (C18H19N3O5S) in the portion of Cefprozil taken:
rU = peak response of the cefprozil (E)-isomer from the Sample solution
rS = peak response of the cefprozil (E)-isomer from Standard solution 1
CS = concentration of USP Cefprozil (E)-Isomer RS in Standard solution 1 (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of the cefprozil (E)-Isomer RS (μg/mg)
Calculate the amount (µg/mg) of cefprozil (C18H19N3O5S) in the portion of Cefprozil taken by adding the values, in µg/mg, of the cefprozil (Z)-isomer and the cefprozil (E)-isomer.
Acceptance criteria: 900–1050 µg/mg on the anhydrous basis
4 IMPURITIES
Organic Impurities, Procedure 1
Use Organic Impurities, Procedure 1 when the impurity prole includes Z-cefprozil open ring, E-cefprozil open ring, and cefprozil related compound K.
Solution A: 11.5 g/L of monobasic ammonium phosphate in water. Adjust, if necessary, with phosphoric acid or ammonium hydroxide to a pH of 4.4.
Solution B: Acetonitrile and Solution A (1:1)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 81 | 19 |
| 8 | 81 | 19 |
| 20 | 36 | 64 |
| 25 | 36 | 64 |
| 27 | 81 | 19 |
| 30 | 81 | 19 |
[Note—These gradient elution times are established on an HPLC system with a dwell volume of approximately 1.3 mL. The gradient elution times in Table 1 can be adjusted as necessary to achieve the separation described.]
Standard stock solution: 0.25 mg/mL each of USP Cefprozil (Z)-Isomer RS, USP Amoxicillin Related Compound I RS, and USP Cefprozil Related Compound D RS in a mixture of 1 M hydrochloric acid and Solution A. Prepare the solution as follows. Dissolve USP Amoxicillin Related Compound I RS, USP Cefprozil (Z)-Isomer RS, and USP Cefprozil Related Compound D RS in 1 M hydrochloric acid, using 20% of the nal volume. Dilute with Solution A to volume.
Sensitivity solution: 2.5 µg/mL each of cefprozil (Z)-isomer, amoxicillin related compound I, and cefprozil related compound D in Solution A from Standard stock solution. Store the solution at 4°, and use within 8 h.
Standard solution: 50 µg/mL each of cefprozil (Z)-isomer, amoxicillin related compound I, and cefprozil related compound D in Solution A from the Standard stock solution. Store the solution at 4°, and use within 12 h.
Sample solution: 5 mg/mL of Cefprozil in a mixture of 1 M hydrochloric acid and Solution A, prepared as follows. Dissolve the Cefprozil rst in 1 M hydrochloric acid using 4% of the nal volume, and then dilute with Solution A to volume. Store the solution at 4°, and use within 3 h. Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Temperatures
Autosampler: 4°
Column: 40°
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: Sensitivity solution and Standard solution
[Note—USP Cefprozil Related Compound D RS contains the (Z)- and (E)-isomers of cefprozil related compound D. See Table 2 for relative retention times.]
Suitability requirements
Resolution: NLT 1.4 between the (E)-isomer of cefprozil related compound D and cefprozil (Z)-isomer, Standard solution
Relative standard deviation: NMT 10.0% for cefprozil, amoxicillin related compound I, and each isomer of cefprozil related compound D, Standard solution
Signal-to-noise ratio: NLT 10 for cefprozil, amoxicillin related compound I, and each isomer of cefprozil related compound D, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of amoxicillin related compound I in the portion of Cefprozil taken:
Result = (rU/rS) × (CS /CU) × P × 100
rU = peak response of amoxicillin related compound I from the Sample solution
rS = peak response of amoxicillin related compound I from the Standard solution
CS = concentration of USP Amoxicillin Related Compound I RS in the Standard solution (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of amoxicillin related compound I in USP Amoxicillin Related Compound I RS (mg/mg)
Calculate the percentage of cefprozil related compound D in the portion of Cefprozil taken:
Result = (rU/rS) × (CS /CU) × P × 100
rU = sum of the responses for cefprozil related compound D (Z)-isomer and cefprozil related compound D (E)-isomer from the Sample solution
rS = peak response of cefprozil related compound D (Z)-isomer from the Standard solutionn
CS = concentration of USP Cefprozil Related Compound D RS in the Standard solution (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of cefprozil related compound D (Z)-isomer in USP Cefprozil Related Compound D RS (mg/mg)
Calculate the percentage of each of the other impurities in the portion of Cefprozil taken:
Result = (rU/rS) × (CS /CU) × P × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of cefprozil from the Standard solution
CS = concentration of USP Cefprozil (Z)-Isomer RS in the Standard solution (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of USP Cefprozil (Z)-Isomer RS (mg/mg)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Amoxicillin related compound Ia | 0.40 | 0.3 |
| Cefadroxil | 0.54 | 0.5 |
| Hydroxyphenyldiketopiperazineb | 0.61 | 0.3 |
| Cefprozil related compound D (Z)-isomerc,d | 0.69 | 0.3 |
| Cefprozil related compound D (E)-isomere | 0.91 | |
| O-Acyl cefprozilf | 0.76 | 0.2 |
| Cefprozil (Z)-isomer | 1.0 | - |
| Cefprozil (E)-isomer | 1.37 | - |
| Z-Cefprozil open ringg | 1.74 | 0.2 |
| Cefprozil related compound H (Z)-isomerh,i | 1.95 | 0.2 |
| Cefprozil related compound H (E)-isomerj | 2.19 | |
| E-Cefprozil open ringk | 2.08 | 0.2 |
| Cefprozil related compound Kl,m | 2.76 | 0.1 |
| 2.86 | 0.1 | |
| 2.91 | 0.1 | |
| 3.01 | 0.1 | |
Any individual unspecified impurity | - | 0.1 |
| Total impurities | - | 2.0 |
a(R)-2-Amino-2-(4-hydroxyphenyl)acetic acid.
b 3-(Aminomethylene)-6-(4-hydroxyphenyl)piperazine-2,5-dione.
c 7-Amino-3-propenylcephalosporanic acid (Z-isomer); (6R,7R)-7-Amino-8-oxo-3-[(Z)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
d The sum of the two isomers is reported. The limit for the sum is 0.3%.
e 7-Amino-3-propenylcephalosporanic acid (E-isomer); (6R,7R)-7-Amino-8-oxo-3-[(E)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
f (6R,7R)-7-[(R)-2-Amino-2-{4-[(R)-2-amino-2-(4-hydroxyphenyl)acetoxy]phenyl}acetamido]-8-oxo-3-[(Z)-prop-1-enyl]-5-thia-1- azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
g(R)-2-{(R)-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido](carboxy)methyl}-5-[(Z)-prop-1-enyl]-3,6-dihydro-2H-1,3-thiazine-4-carboxylic acid. h N-Acyl cefprozil (Z-isomer); (6R,7R)-7-{(R)-2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-(4-hydroxyphenyl)acetamido}-8-oxo-3-[(Z)- prop-1-enyl]-5-thia-1 azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
i The sum of the two isomers is reported. The limit for the sum is 0.2%.
j N-Acyl cefprozil (E-isomer); (6R,7R)-7-{(R)-2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-(4-hydroxyphenyl)acetamido}-8-oxo-3-[(E)- prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
k(R)-2-{(R)-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido](carboxy)methyl}-5-[(E)-prop-1-enyl]-3,6-dihydro-2H-1,3-thiazine-4-carboxylic acid. l Hydroxyphenyldiketopiperazine lactone; 3-(5-Ethyl-7-oxo-2,4,5,7-tetrahydro-1H-furo[3,4-d][1,3]thiazin-2-yl)-6-(4-hydroxyphenyl)piperazine 2,5-dione.
m The system resolves four isomers of cefprozil related compound K.
Organic Impurities, Procedure 2
Use Organic Impurities, Procedure 2 when the impurity prole includes ethoxycarbonyl cefprozil, methoxycefadroxil, cefprozil delta-3 isomer, cefprozil amide, and cefprozil dimer.
Solution A: 4 g/L of monobasic sodium phosphate adjusted with dilute phosphoric acid (1 in 10) to a pH of 4.2 ± 0.05 Solution B: Acetonitrile and Solution A (1:1)
Mobile phase: See Table 3.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 95 | 5 |
| 20 | 70 | 30 |
| 40 | 40 | 60 |
| 50 | 0 | 100 |
| 60 | 0 | 100 |
| 62 | 95 | 5 |
| 70 | 95 | 5 |
Diluent: 0.85 g/L of monobasic potassium phosphate and 1.16 g/L of anhydrous dibasic sodium phosphate in water
System suitability stock solution: 0.15 mg/mL of USP Cefadroxil RS and 0.75 mg/mL of USP Cefprozil Related Compound D RS, prepared as follows. Dissolve USP Cefadroxil RS in Solution A, using 20% of the nal volume. Add USP Cefprozil Related Compound D RS, mix, and dilute with Diluent to volume.
System suitability solution: 15 µg/mL of USP Cefadroxil RS and 75 µg/mL of USP Cefprozil Related Compound D RS from the System suitability stock solution and 1.5 mg/mL of USP Cefprozil RS in Solution A
Standard solution: 15 µg/mL of USP Cefprozil RS in Solution A
Sample solution: 1.5 mg/mL of Cefprozil in Solution A. Refrigerate the solution, and use within 1 h.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Temperatures
Autosampler: 4°
Column: NMT 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between the (Z)-isomer of cefprozil related compound D and cefadroxil; NLT 1.5 between cefadroxil and the (E)- isomer of cefprozil related compound D, System suitability solution
Relative standard deviation: NMT 5.0% for the sum of the cefprozil (Z)-isomer and cefprozil (E)-isomer, Standard solution Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Cefprozil taken:
Result = (rU/rS) × (CS /CU) × P × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = sum of the responses for cefprozil (Z)-isomer and cefprozil (E)-isomer from the Standard solution
CS = concentration of USP Cefprozil RS in the Standard solution (mg/mL)
CU = concentration of Cefprozil in the Sample solution (mg/mL)
P = potency of USP Cefprozil RS (mg/mg)
F = relative response factor (see Table 4)
Acceptance criteria: See Table 4. The reporting threshold is 0.05%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Amoxicillin related compound Ia | 0.17 | 1.5 | 0.15 |
| Cefprozil related compound D (Z)-isomerb | 0.57 | 0.56 | 0.30 |
| Cefadroxil | 0.62 | 1.1 | 1.0 |
| Methoxycefadroxilc | 0.65 | 0.44 | 0.15 |
| Cefprozil related compound D (Z)-isomer,d | 0.73 | 0.56 | 0.30 |
| Cefprozil delta-3 isomere | 0.92 | 0.95 | 0.2 |
| Cefprozil (Z)-isomer | 1.0 | - | - |
| Cefprozil (E)-isomer | 1.17 | - | - |
| Cefprozil related compound Hf | 1.33 | 0.93 | 0.15 |
| Cefprozil amideg | 1.46 | 0.90 | 0.15 |
| Ethoxycarbonylcefprozilh | 2.08 | 0.70 | 0.15 |
| Cefprozil dimeri | 2.21 | 0.90 | 0.2 |
Any individual unspecified impurity | - | 1.0 | 0.2 |
| Total impurities | - | - | 2.0 |
a(R)-2-Amino-2-(4-hydroxyphenyl)acetic acid.
b 7-Amino-3-propenylcephalosporanic acid (Z-isomer); (6R,7R)-7-Amino-8-oxo-3-[(Z)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
c(6R,7R)-7-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. d 7-Amino-3-propenylcephalosporanic acid (E-isomer); (6R,7R)-7-Amino-8-oxo-3-[(E)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
e(6R,7R)-7-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-8-oxo-3-[(Z)-prop-1-en-1-yl]-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid. f N-Acyl cefprozil (Z-isomer); (6R,7R)-7-{(R)-2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-(4-hydroxyphenyl)acetamido}-8-oxo-3-[(Z)- prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
g(R)-2-{(6R,7R)-7-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-8-oxo-3-[(Z)-prop-1-en-1-yl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxamido}-2-(4-hydroxyphenyl)acetic acid.
h(6R,7R)-7-{(R)-2-Amino-2-[4-(ethoxycarbonyloxy)phenyl]acetamido}-8-oxo-3-[(Z)-prop-1-en-1-yl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid.
i (6R,7R)-7-[(R)-2-{(6R,7R)-7-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-8-oxo-3-[(Z)-prop-1-en-1-yl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxamido}-2-(4-hydroxyphenyl)acetamido]-8-oxo-3-[(Z)-prop-1-en-1-yl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
5 SPECIFIC TESTS
Crystallinity 〈695〉: Meets the requirements
pH 〈791〉
Sample solution: 5 mg/mL in water
Acceptance criteria: 3.5–6.5
Water Determination 〈921〉, Method I: 3.5%–6.5%
Cefprozil (E)-Isomer Ratio
Buffer, Mobile phase, System suitability solution, Standard solution 1, Standard solution 2, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Samples: Standard solution 1, Standard solution 2, and Sample solution
Calculate the ratio of the cefprozil (E)-isomer to total cefprozil in the portion of Cefprozil taken:
Result = E/(E + Z)
E = amount of cefprozil (E)-isomer as determined in the Assay (µg/mg)
Z = amount of cefprozil (Z)-isomer as determined in the Assay (µg/mg)
Acceptance criteria: The ratio is 0.06–0.11.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: If a test for Organic Impurities other than Procedure 1 is used, then the labeling states with which Organic Impurities test the article complies.
USP Reference Standards 〈11〉
USP Amoxicillin Related Compound I RS
(R)-2-Amino-2-(4-hydroxyphenyl)acetic acid.
C6H9N2O3 167.16
USP Cefadroxil RS
USP Cefprozil RS
USP Cefprozil (E)-Isomer RS
USP Cefprozil (Z)-Isomer RS
USP Cefprozil Related Compound D RS
7-Amino-3-propenylcephalosporanic acid;
(6R,7R)-7-Amino-8-oxo-3-[(Z)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
C10H12N2O3S 240.28


