Cefoxitin Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
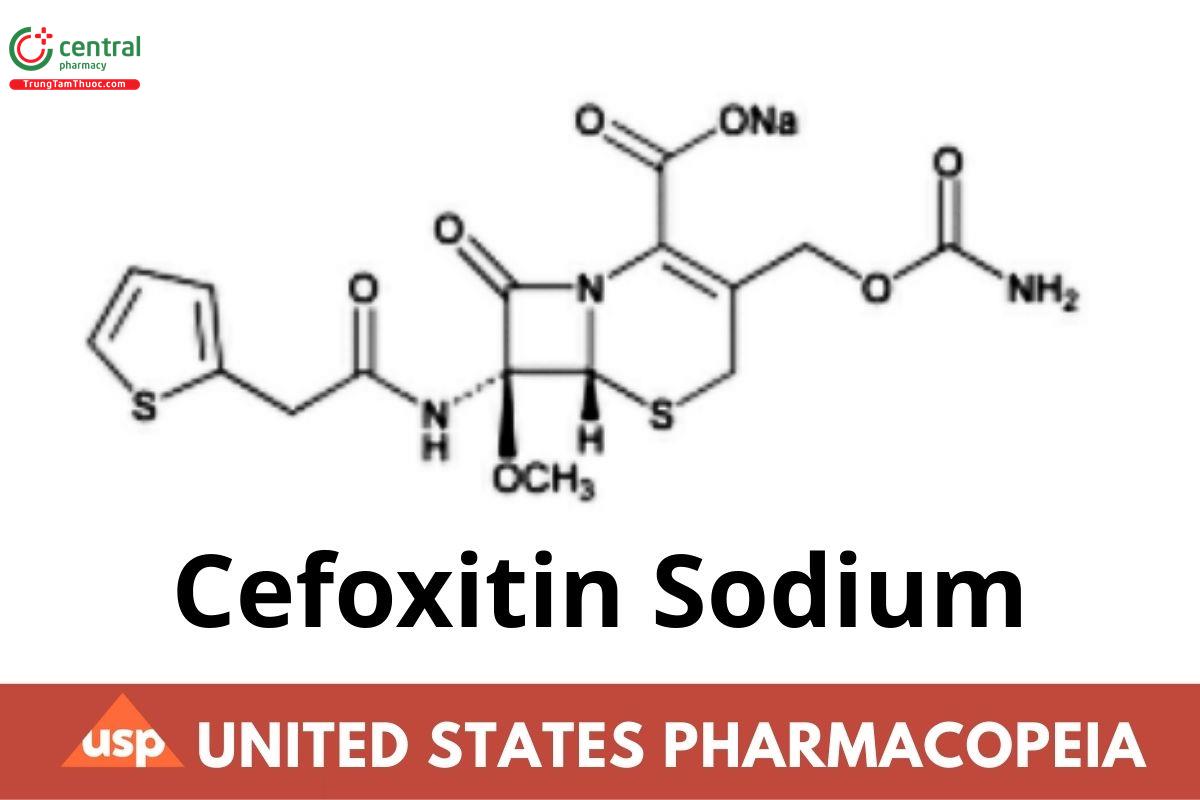
C16H16N3NaO7S2. 449.43
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-, sodium salt (6R-cis)-;
Sodium (6R,7S)-3-(hydroxymethyl)-7-methoxy-8-oxo-7-[2-(2-thienyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate carbamate (ester) CAS RN®: 33564-30-6; UNII: Q68050H03T.
1 DEFINITION
Cefoxitin Injection contains the equivalent of NLT 927 μg/mg and NMT 970 μg/mg of cefoxitin (C16H17N3O7S2), corresponding to NLT 97.5% and NMT 102.0% of cefoxitin sodium (C16H16N3O7S2), calculated on the anhydrous and acetone- and methanol-free basis.
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Buffer: 1.0 g/L of monobasic potassium phosphate and 1.8 g/L of anhydrous dibasic sodium phosphate in water
Sample solution: 20 μg/mL in Buffer
Acceptance criteria: Meets the requirements
C. Identification Tests—General, Sodium〈191〉
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Buffer: Dissolve 1.0 g of monobasic potassium phosphate and 1.8 g of dibasic sodium phosphate in 900 mL of water. Adjust with phosphoric acid or 10 N sodium hydroxide to a pH of 7.1 ± 0.1, and dilute with water to 1 L. Pass through a membrane filter of 1-µm or finer pore size.
Mobile phase: Acetonitrile, water, and glacial acetic acid (160:840:10). Pass through a membrane filter of 1-µm or finer pore size.
Standard solution: 0.3 mg/mL of USP Cefoxitin RS in Buffer. Sonicate, if necessary, to dissolve. Use this solution within 5 h.
Sample solution: 0.3 mg/mL of cefoxitin prepared as follows. Allow one container of Injection to thaw, and mix. Dilute an aliquot of Injection with Buffer to a suitable volume. Use this solution within 5 h.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm × 30-cm; 5- to 10-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 2800 theoretical plates
Tailing factor: NMT 1.5
Relative standard deviation: NMT 1.0%
Analysis
Samples: Sample solution 1 or Sample solution 2 and Standard solution
Calculate the percentage of the labeled amount of cefoxitin (C16H17N3O7S2) in the portion of Cefoxitin for Injection taken:
Result = (rU/rS) × (CS/CU) × P × F
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
P = potency of cefoxitin in USP Cefoxitin RS (mg/mg)
F = conversion factor, 1000 μg/mg
Acceptance criteria: 927–970 μg/mg of cefoxitin, corresponding to NLT 97.5% and NMT 102.0% of cefoxitin sodium, on the anhydrous and acetone- and methanol-free basis
4 IMPURITIES
Limit of Acetone and Methanol
Standard stock solution 1: 0.5% v/v of acetone in water
Standard stock solution 2: 0.5% v/v of methanol in water
Standard solution: 0.050% v/v of acetone from Standard stock solution 1 and 0.005% v/v of methanol from Standard stock solution 2 in water Sample solution: Transfer 5.0 g of Cefoxitin Sodium to a 50-mL volumetric flask, and dissolve in and dilute with water to volume. Transfer 3.0 mL of the resulting solution to a 15-mL centrifuge tube, cool in an ice-water bath for 2 min, and add 3.0 mL of 0.24 N hydrochloric acid while swirling vigorously. Centrifuge to obtain a clear solution.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization Columns
Precolumn: Packed with 60- to 80-mesh silane-treated glass beads
Analytical: 6.3-mm × 1.8-m glass; support S2
Temperatures
Columns: 110°
Injection port: 100°
Detector: 200°
Carrier gas: Nitrogen
Flow rate: 50 mL/min
Injection volume: 2 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 160 theoretical plates for acetone and NLT 200 theoretical plates for methanol Tailing factor: NMT 1.3 for acetone and NMT 2.3 for methanol
Relative standard deviation: NMT 5%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentages of acetone and methanol in the portion of Cefoxitin Sodium taken:
Result = (rU/rS) × (CS/CU) × D
rU = peak area of acetone or methanol in the Sample solution
rS = peak area of acetone or methanol in the Standard solution
CS = concentration of acetone or methanol in the Standard solution (% v/v)
CU = concentration of Cefoxitin Sodium in the Sample solution (g/mL)
D = density of acetone or methanol at 20° (g/mL)
Acceptance criteria: NMT 0.7% of acetone and NMT 0.1% of methanol
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL in methanol
Acceptance criteria: +206° to +214°, calculated on the anhydrous and acetone- and methanol-free basis
Crystallinity 〈695〉: Meets the requirements
pH 〈791〉
Sample solution: 100 mg/mL
Acceptance criteria: 4.2–7.0
Water Determination, Method I〈921〉
Analysis: Use a mixture of ethylene glycol and pyridine (3:1) in the titration vessel in place of methanol.
Acceptance criteria: NMT 1.0%
Bacterial Endotoxins Test 〈85〉: Where the label states that Cefoxitin Sodium is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.13 USP Endotoxin Unit/mg of cefoxitin.
Sterility Tests 〈71〉: Where the label states that Cefoxitin Sodium is sterile, it meets the requirements when tested as directed in Test for Sterility of the Product to Be Examined, Membrane Filtration.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store in a cold place.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Cefoxitin RS


