Cefmetazole Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
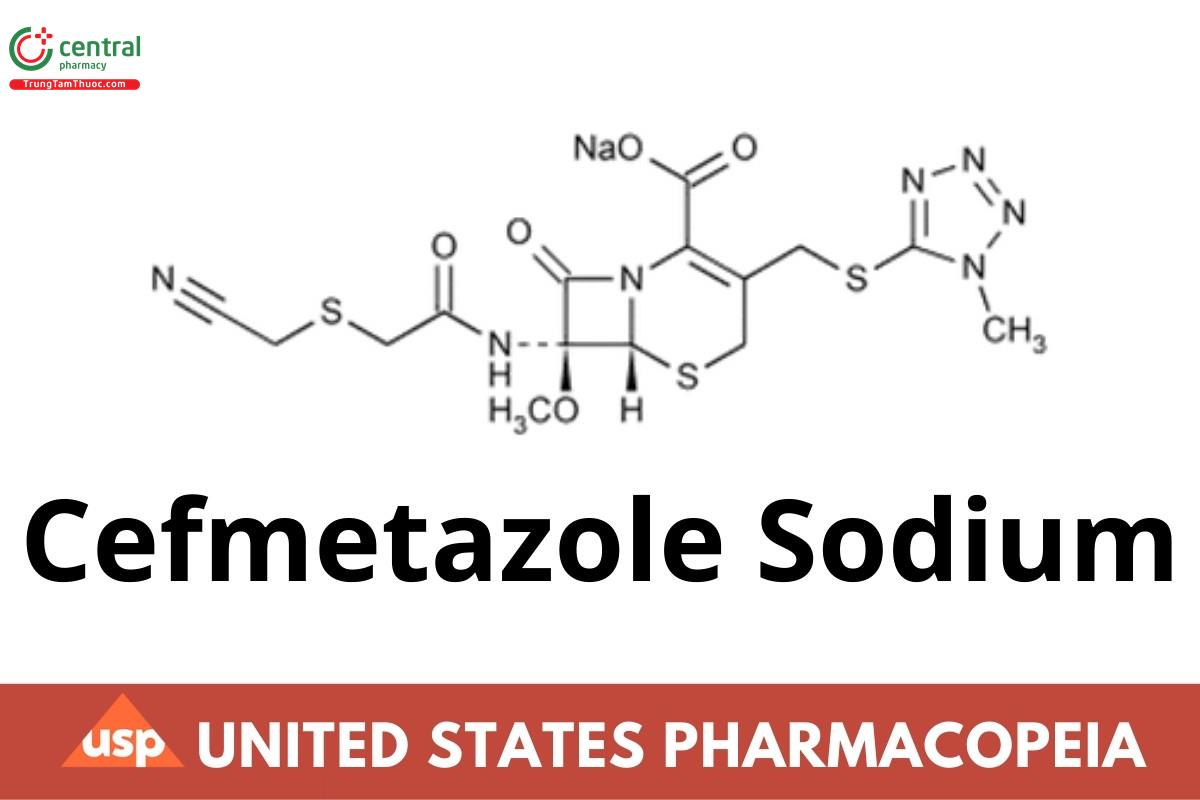
Cefmetazole Sodium contains the equivalent of not less than 860 µg and not more than 1003 µg of cefmetazole (C15H17N7O5S3) per mg, calculated on the anhydrous basis.
Packaging and storage—Preserve in tight containers.
Labeling—Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP REFERENCE STANDARDS 〈11〉—
USP Cefmetazole RS
1 Identification—
Change to read:
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 19 7M (CN 1-May-2020) .
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
PH 〈791〉: between 4.2 and 6.2, in a solution (1 in 10). WATER DETERMINATION, Method I 〈921〉: not more than 0.5%.
Other requirements —Where the label states that Cefmetazole Sodium is sterile, it meets the requirements in the tests for Sterility Tests 〈71〉 and for Bacterial endotoxins under Cefmetazole for Injection. Where the label states that Cefmetazole Sodium must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements in the test for Bacterial endotoxins under Cefmetazole for Injection.
2 Assay—
Mobile phase, Standard preparation, Resolution solution, and Chromatographic system—Proceed as directed in the Assay under Cefmetazole. Assay preparation—Transfer about 21 mg of Cefmetazole Sodium, accurately weighed, to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix. [NOTE—Use this solution within 10 minutes.]
Procedure— Proceed as directed in the Assay under Cefmetazole. Calculate the quantity, in µg, of cefmetazole (C15H17N7O5S3) per mg of Cefmetazole Sodium taken by the formula:
100(C/M)(ru/rs)
in which C is the concentration, in µg per mL, of cefmetazole (C15H17N7O5S3) in the Standard preparation; M is the quantity, in mg, of Cefmetazole Sodium taken to prepare the Assay preparation; and r and r are the cefmetazole peak responses obtained from the Assay preparation and the Standard preparation, respectively.


