Cefepime Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
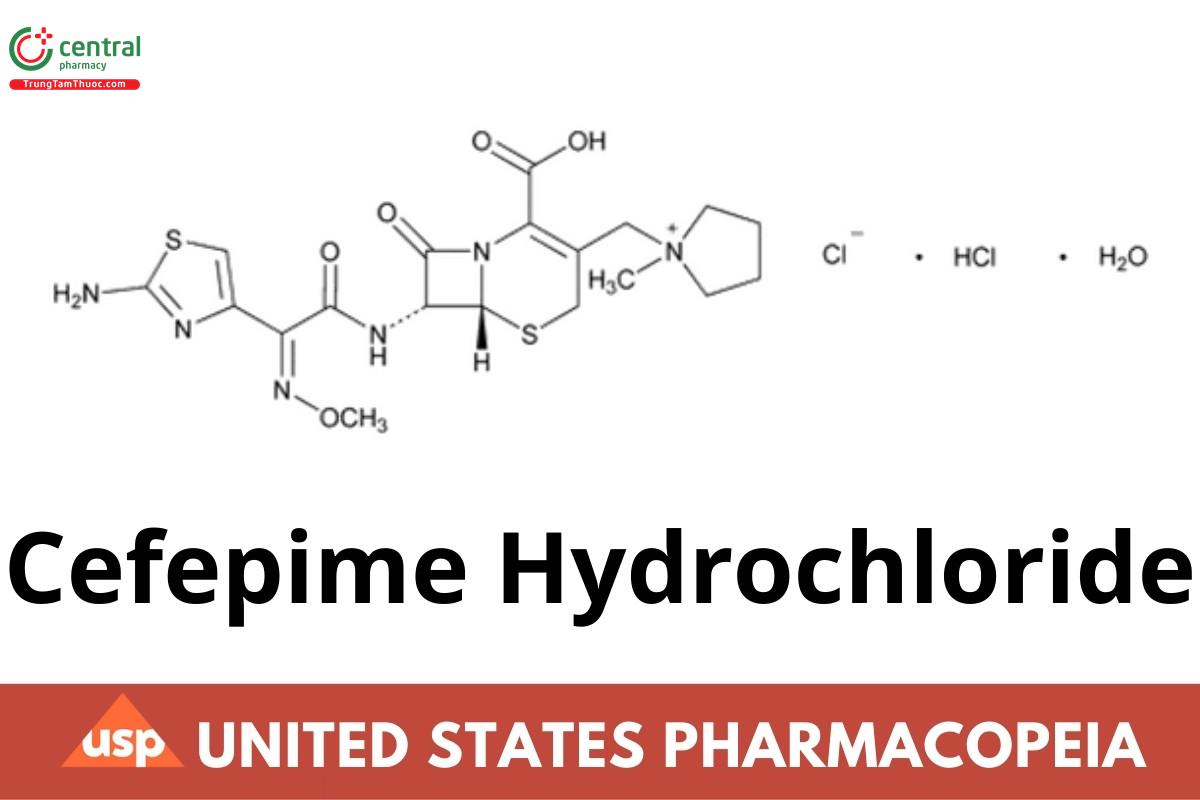
1 DEFINITION
Cefepime Hydrochloride contains the equivalent of NLT 825 µg/mg and NMT 911 µg/mg of cefepime (C19H24N6O5S2), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS 〈197〉 , Infrared Spectroscopy: ▲197A, 197K, or▲ (USP 1-May-2024) 197M
Sample: Proceed as directed in the chapter, but do not dry. Acceptance criteria: Meets the requirements
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. Add the following:
C. IDENTIFICATION TESTS—GENERAL 〈191〉 , Chemical Identification Tests, Chloride: Meets the requirements▲ (USP 1-May-2024)
3 ASSAY
Change to read:
PROCEDURE
Solution A: 0.68 mg/mL of monobasic potassium phosphate in water
Solution B: Acetonitrile and Solution A (10:90). Adjust with 2% phosphoric acid or 2% potassium hy droxide to a pH of 5.0. Solution C: Acetonitrile and Solution A (50:50). Adjust with 2% phosphoric acid or 2% potassium hydroxide to a pH of 5.0. Mobile phase: See Table 1.


