Cefdinir
If you find any inaccurate information, please let us know by providing your feedback here
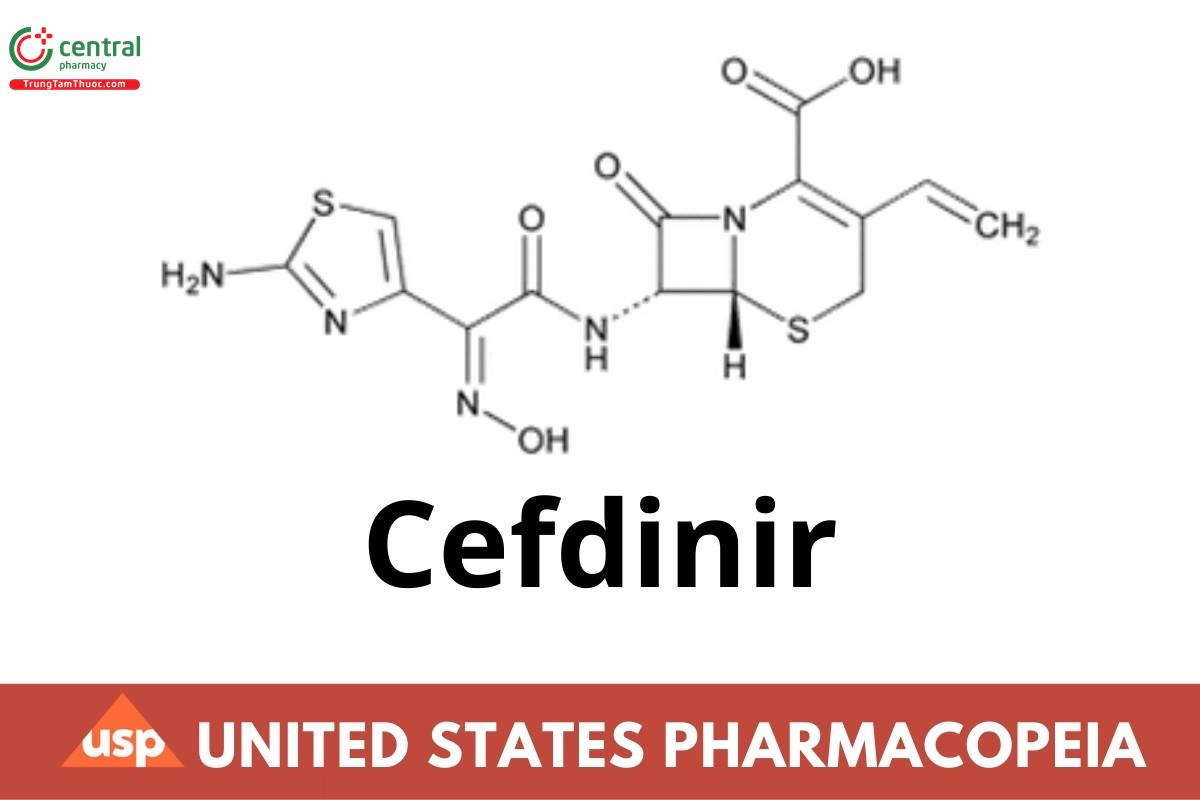
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Cefdinir contains NLT 940 µg/mg and NMT 1030 µg/mg of cefdinir (C14H13N5O5S2), calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS 〈197〉 , Infrared Spectroscopy : 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Solution A: 14.2 g/L of anhydrous dibasic sodium phosphate Solution B: 13.6 g/L of monobasic potassium phosphate
Solution C: Dilute tetramethylammonium hydroxide (10%) with water to obtain a 0.1% solution. Adjust with 10% phosphoric acid to a pH of 5.5.
Solution D: 37.2 mg/mL of edetate disodium
Buffer: Combine appropriate amounts of Solution A and Solution B (about 2:1) to obtain a solution with a pH of 7.0. Mobile phase: Acetonitrile, methanol, Solution C, and Solution D (300:200:4500:2)
System suitability solution: 0.2 mg/mL of USP Cefdinir RS and 0.5 mg/mL of USP Cefdinir Related Compound A RS in Buffer Standard solution: 0.2 mg/mL of USP Cefdinir RS in Buffer
Sample solution: 0.2 mg/mL of Cefdinir in Buffer
3.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1 Column temperature: 40°
Flow rate: 1 mL/min
Injection size: 5 µL System suitability
Samples: System suitability solution and Standard solution. USP Cefdinir Related Compound A RS should produce four peaks.
Tailing factor: NMT 1.5 for cefdinir, System suitability solution
Resolution: NLT 1.2 between the second peak of cefdinir related compound A and cefdinir, System suitability solution Relative standard deviation: NMT 1.0%, Standard solution
3.1.2 Analysis
Samples: Standard solution and Sample solution
Calculate the quantity, in µg/mg, of cefdinir (C H N O S ) in the portion of Cefdinir taken:
Result = (ru/rs) × (Cs/Cu) × P
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of the Standard solution (mg/mL)
Cu = concentration of the Sample solution (mg/mL)
P = purity of USP Cefdinir RS (µg/mg)
Acceptance criteria: 940–1030 µg/mg on the anhydrous basis
4 IMPURITIES
RESIDUE ON IGNITION 〈281〉: NMT 0.20%
Change to read:
ORGANIC IMPURITIES
Solution A, Solution B, Solution C, Solution D, and Buffer: Prepare as directed in the Assay. Solution E: To 1000 mL of Solution C add 0.4 mL of Solution D.
Solution F: Acetonitrile, methanol, Solution C, and Solution D (300:200:500:0.4) Mobile phase: See Table 1.


