Carbidopa
If you find any inaccurate information, please let us know by providing your feedback here
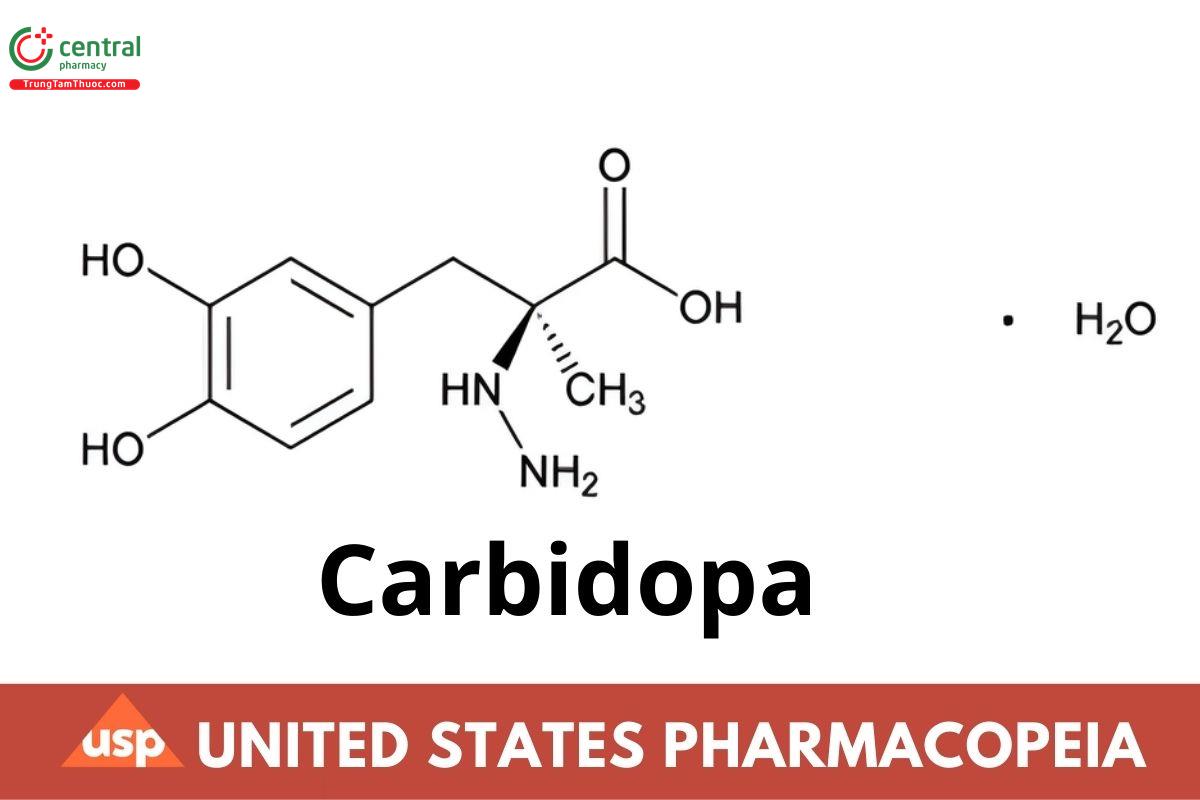
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C10H14N2O4 · H2O 244.24
C10H14N2O4 226.23
Benzenepropanoic acid, α-hydrazino-3,4-dihydroxy-α-methyl-, monohydrate, (S)-;
(−)-l-α-Hydrazino-3,4-dihydroxy-α-methylhydrocinnamic acid monohydrate CAS RN®: 38821-49-7; UNII: MNX7R8C5VO.
Anhydrous CAS RN®: 28860-95-9; UNII: KR87B45RGH.
1 DEFINITION
Carbidopa contains NLT 98.0% and NMT 102.0% of carbidopa (C10H14N2O4 · H2O).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Buffer: 0.05 M monobasic sodium phosphate, adjusted with phosphoric acid to a pH of 2.7
Mobile phase: Alcohol and Buffer (5:95)
System suitability solution: 0.1 mg/mL of USP Carbidopa RS and 0.1 mg/mL of USP Methyldopa RS in Mobile phase
Standard solution: 0.5 mg/mL of USP Carbidopa RS in Mobile phase. [Note—Use gentle heat and ultrasonification, if necessary, to dissolve.]
Sample solution: 0.5 mg/mL of Carbidopa in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 3.9-mm × 30-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 20 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for methyldopa and carbidopa are about 0.8 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 0.9 between methyldopa and carbidopa, System suitability solution
Relative standard deviation: NMT 1.5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration, in mg/mL, of carbidopa (C10H14N2O4 · H2O) in the Standard solution (CS):
Result = CS2 × (Mr1/Mr2)
CS2 = concentration of USP Carbidopa RS, as determined using the value on the USP Reference Standard label, in the Standard solution (mg/mL)
Mr1 = molecular weight of carbidopa monohydrate, 244.24
Mr2 = molecular weight of anhydrous carbidopa, 226.23
Calculate the percentage of carbidopa (C10H14N2O4 · H2O) in the portion of Carbidopa taken:
Result = (rU/rS) x (CS/CU ) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of carbidopa (C10H14N2O4 · H2O) in the Standard solution (mg/mL)
CU = concentration of Carbidopa in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Limit of Methyldopa and Carbidopa Related Compound A
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Impurity standard solution: 2.5 μg/mL of USP Methyldopa RS and 2.5 μg/mL of USP Carbidopa RS in Mobile phase
Analysis
Samples: Sample solution and Impurity standard solution
[Note—The relative retention times for methyldopa, carbidopa, and carbidopa related compound A are about 0.8, 1.0, and 1.8, respectively.]
Calculate the percentage of methyldopa in the portion of Carbidopa taken:
Result = (rU/rS) x (CS/CU ) × 100
rU = peak response of methyldopa from the Sample solution
rS = peak response of methyldopa from the Impurity standard solution
CS = concentration of USP Methyldopa RS in the Impurity standard solution (μg/mL)
CU = concentration of the Sample solution (μg/mL)
Calculate the percentage of carbidopa related compound A in the portion of Carbidopa taken:
Result = (rU/rS) x (CS/CU ) × 100
rU = peak response of carbidopa related compound A from the Sample solution
rS = peak response of carbidopa from the Impurity standard solution
CS = concentration of USP Carbidopa RS in the Impurity standard solution (μg/mL)
CU = concentration of the Sample solution (μg/mL)
Acceptance criteria: NMT 0.5% of methyldopa and NMT 0.5% of carbidopa related compound A
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL in 0.7 g/mL of aluminum chloride solution (prepared using the hexahydrate form of the aluminum salt) that has been filtered and adjusted with 0.25 N sodium hydroxide to a pH of 1.5
Acceptance criteria: −21.0° to −23.5° calculated as the monohydrate
Loss on Drying 〈731〉
Analysis: Heat 1 g in a suitable vacuum drying apparatus at 100° and a pressure of NMT 5 mm of mercury to constant weight. Cool, and weigh.
Acceptance criteria: 6.9%–7.9%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.
USP Reference Standards 〈11〉
USP Carbidopa RS
USP Methyldopa RS


