Carbamide Peroxide
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
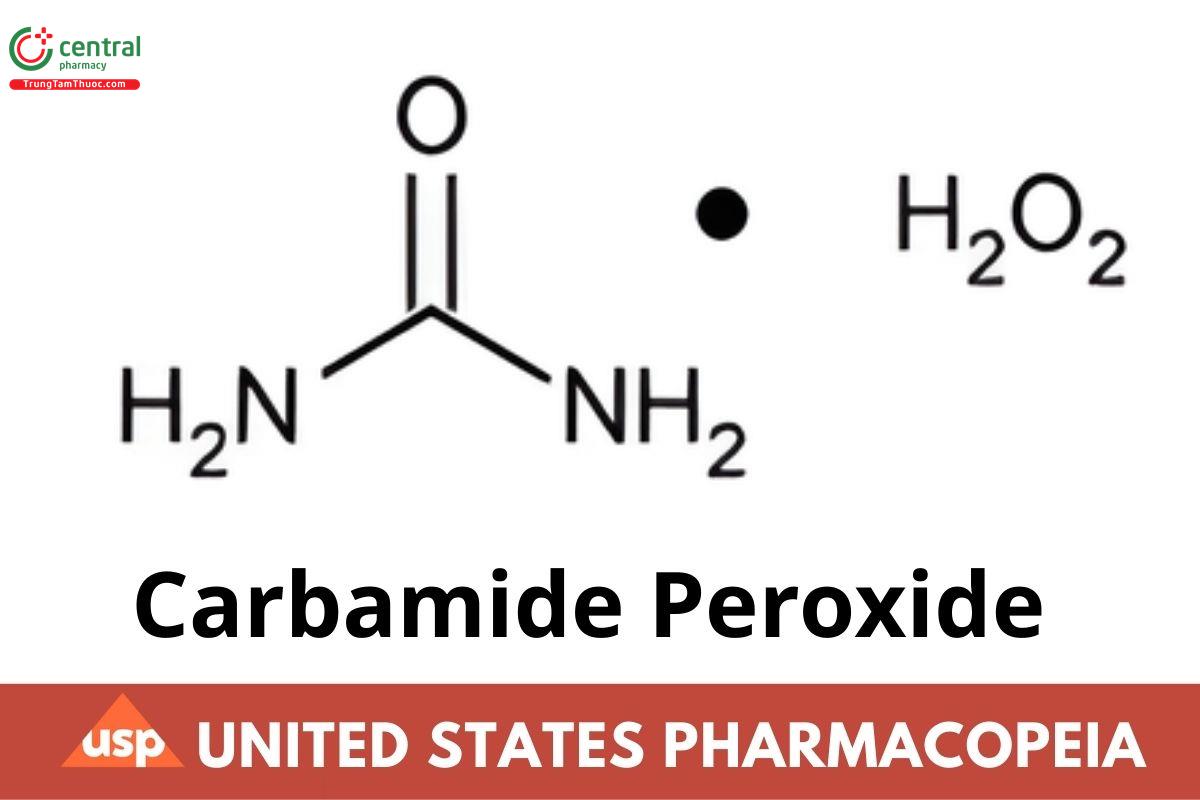
CH6ON2O3 94.07
Urea, compd. with Hydrogen peroxide (1:1).
Urea compound with hydrogen peroxide (1:1) CAS RN®: 124-43-6; UNII: 31PZ2VAU81.
Carbamide Peroxide contains not less than 96.0 percent and not more than 102.0 percent of CH6ON2O3.
Packaging and storage-Preserve in tight, light-resistant containers, and avoid exposure to excessive heat.
Identification-
A: Mix 1 mL of a solution (1 in 10) of it with 1 mL of nitric acid: a white, crystalline precipitate is formed.
B: A solution of it (1 in 10) responds to the tests for Peroxide (191).
Assay-Transfer about 100 mg of Carbamide Peroxide, accurately weighed, to a 500-mL iodine flask with the aid of 25 mL of water, add 5 mL of glacial acetic acid, and mix. Add 2 g of potassium iodide and 1 drop of ammonium molybdate TS, insert the stopper, and allow to stand in the dark for 10 minutes. Titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Each mL of 0.1 N sodium thiosulfate is equivalent to 4.704 mg of CH6ON2O3.


