Carbamazepine Extended-Release Tablets
If you find any inaccurate information, please let us know by providing your feedback here

This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
To view the Notice from the Expert Committee that posted in conjunction with this accelerated revision, please click www.uspnf.com/rb-carbamazepine-ert-20240628.
1 DEFINITION
Carbamazepine Extended-Release Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of carbamazepine (C15H12N2O).
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Standard solution: 10 μg/mL of USP Carbamazepine RS in methanol
Sample solution: Finely powder 1 Tablet, and quantitatively transfer the powder, with the aid of methanol, to a 100-mL volumetric flask. Add about 70 mL of methanol, and shake by mechanical means for 60 min. Sonicate for 15 min, and dilute with methanol to volume. Allow to stand for 10–15 min. Dilute a portion of the clear solution with methanol to obtain a solution containing about 10 μg/mL of carbamazepine. Acceptance criteria: Meet the requirements
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Methanol, methylene chloride, and water (450:45:600)
Internal standard solution: 600 μg/mL of Phenytoin in methanol
Standard stock solution: 200 μg/mL of USP Carbamazepine RS in methanol
Standard solution: 100 μg/mL of carbamazepine from Standard stock solution in Internal standard solution
System suitability solution: 50 μg/mL of carbamazepine from Standard solution in Internal standard solution
Sample stock solution A: Nominally 4 mg/mL of carbamazepine from finely powdered Tablets prepared as follows. Finely powder 10 Tablets.
Transfer the powder to an appropriate volumetric flask with the aid of methanol. Add 70% of the flask volume of methanol. Shake by mechanical means for 60 min. Sonicate for 15 min, and dilute with methanol to volume. Allow to stand for 10–15 min, and then filter a portion of the supernatant. Use the clear filtrate.
Sample stock solution B: Nominally 0.2 mg/mL of carbamazepine from Sample stock solution A in methanol
Sample solution: Nominally 100 μg/mL of carbamazepine from Sample stock solution B in Internal standard solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Columns
Guard: 4.6-mm × 30-mm; 7-μm packing L7
Analytical: 3.9-mm × 30-cm; packing L1
Flow rate: 2 mL/min
Injection volume: 10 μL
System suitability
Sample: System suitability solution
[Note—The relative retention times for phenytoin and carbamazepine are about 0.8 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.8 between phenytoin and carbamazepine
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) in the portion of Tablets taken:
Result = (RU/RS ) × (CS/CU) × 100
Ru = peak response ratio of carbamazepine to the internal standard from the Sample solution
Rs = peak response ratio of carbamazepine to the internal standard from the Standard solution
Cs = concentration of USP Carbamazepine RS in the Standard solution (μg/mL)
Cu= nominal concentration of carbamazepine in the Sample solution (μg/mL)
Acceptance criteria: 90.0%–110.0%
4 PERFORMANCE TESTS
Change to read:
Dissolution 〈711〉
Test 1
Medium
For Tablets labeled to contain 100 mg or 200 mg: Water; 900 mL
For Tablets labeled to contain 400 mg: Water; 1800 mL
Apparatus 1: 100 rpm
Times: 3, 6, 12, and 24 h
Standard solution: USP Carbamazepine RS in Medium
Sample solution: Filtered portions of the solution under test, diluted with Medium if necessary
Instrumental conditions
Mode: UV
Analytical wavelength: The wavelength of maximum absorbance at about 284 nm
Analysis
Samples: Standard solution and Sample solution
Determine the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time using the UV absorption.
Tolerances: See Table 1.
Table 1
| Time (h) | Amount Dissolved |
| 3 | 10%-35% |
| 6 | 35%-65% |
| 12 | 65%-90% |
| 24 | NLT 75% |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 2: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL
For Tablets labeled to contain 400 mg: Water; 1800 mL
Apparatus 2: 100 rpm, with sinkers
Times: 2, 4, 12, and 24 h
Standard stock solution: 0.55 mg/mL of USP Carbamazepine RS in methanol. Sonication may be used to promote dissolution.
Standard solution: 0.0088 mg/mL of USP Carbamazepine RS from Standard stock solution in Medium
Sample stock solution: Pass a portion of the solution under test through a suitable filter of 0.45-μm pore size. Discard the first 3 mL of the filltrate. Replace the portion removed from the solution under test with the same volume of Medium.
Sample solution
For Tablets labeled to contain 100 mg: Transfer 2.0 mL of Sample stock solution to a 25-mL volumetric flask and dilute with Medium to volume.
For Tablets labeled to contain 200 or 400 mg: Transfer 2.0 mL of Sample stock solution to a 50-mL volumetric flask and dilute with Medium to volume.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 284 nm
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Result = (AU /AS ) × CS x D
AU = absorbance from the Sample solution at time point i
AS = absorbance from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2xV2) + (C1xVS) x (1/L) x 100
Result3 = {(C3 × V) + [(C1 + C2 ) × VS]} × (1/L) × 100
Result4 = {(C4 × V) + [(C1 + C2 + C3 ) × VS]} × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
Vs = volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 2.
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Table 2
| Time Point (i) | Time (h) | Amount Dissolved (for Tablets that contain 100 mg of carbamazepine) (%) | Amount Dissolved (for Tablets that contain 200 or 400 mg of carbamazepine) (%) |
| 1 | 2 | 10-30 | 10-30 |
| 2 | 4 | 42-62 | 35-55 |
| 3 | 12 | 68-88 | 68-88 |
| 4 | 24 | NLT 70 | NLT 70 |
Test 3: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 3.
Medium: 5 g/L of sodium dodecyl sulfate in water; 900 mL, deaerated if necessary
Apparatus 1: 100 rpm, with a fixture made of 316 stainless steel to prevent the Tablets from turning during the test (see Figure 1).
For Tablets with a release hole, orient the hole facing downward in the basket.
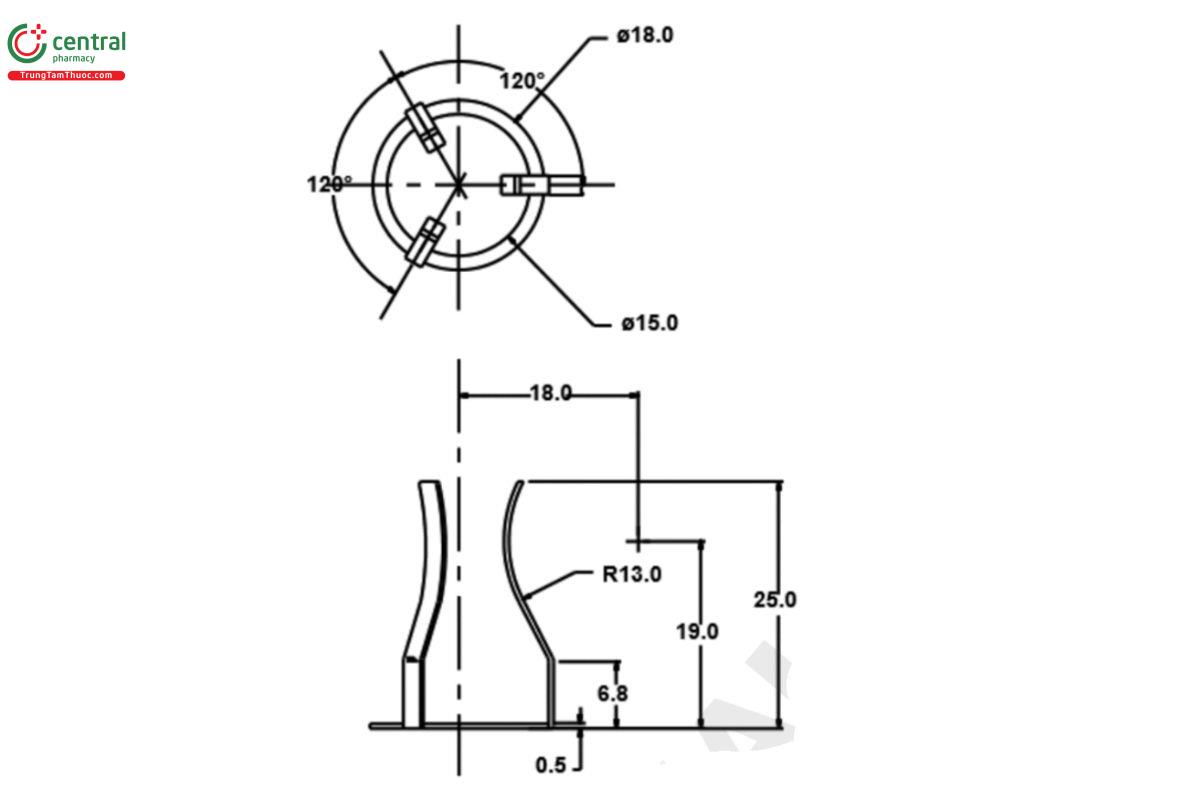
Times: 3, 6, 12, and 24 h
Standard stock solution: 0.22 mg/mL of USP Carbamazepine RS prepared as follows. Weigh a suitable amount of USP Carbamazepine RS in a suitable volumetric flask. Add methanol to 10% of the flask volume and shake for 10 min to dissolve. Dilute with water to volume.
Standard solution: 0.0088 mg/mL of USP Carbamazepine RS from Standard stock solution in Medium
Sample solution: At the specified time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of 0.45- μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained. Dilute with Medium to a concentration similar to that of the Standard solution.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 284 nm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Result = (A U/AS ) × CS × D
AU = absorbance from the Sample solution at time point i
AS = absorbance from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = {[C2 × (V − VS)] + (C1 × VS)} × (1/L) × 100
Result3 = ({C3 × [V − (2 × VS)]} + [(C2 + C1 ) × VS ]) × (1/L) × 100
Result4 = ({C4 × [V − (3 × VS)]} + [(C3 + C2 + C1 ) × VS]) × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of the Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point from the Medium (mL)
Tolerances: See Table 3.
Table 3
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 3 | 15-40 |
| 2 | 6 | 42-67 |
| 3 | 12 | 65-85 |
| 4 | 24 | NLT 75 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 4: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 4.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL
For Tablets labeled to contain 400 mg: Water; 1800 mL
Apparatus 1: 100 rpm
For Tablets with a release hole, orient the hole facing downward in the basket.
Times: 3, 6, 12, and 24 h
Standard stock solution: 0.22 mg/mL of USP Carbamazepine RS prepared as follows. Transfer a suitable amount of USP Carbamazepine
RS to a suitable volumetric flask. Add methanol to 5% of the ask volume. Sonicate to dissolve. Dilute with Medium to volume.
Standard solution: 0.011 mg/mL of USP Carbamazepine RS from Standard stock solution in Medium
Sample stock solution: At the specified time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of
0.45-μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained. Replace the portion removed from the solution under test with the same volume of Medium.
Sample solution
For Tablets labeled to contain 100 mg: Dilute 5 mL of Sample stock solution to 50 mL with Medium.
For Tablets labeled to contain 200 or 400 mg: Dilute 5 mL of Sample stock solution to 100 mL with Medium.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 284 nm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Resulti = (AU/AS) × CS × D
AU = absorbance from the Sample solution at time point i
AS = absorbance from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2xV2) + (C1xVS) x (1/L) x 100
Result3 = {(C3 × V) + [(C1 + C2 ) × VS]} × (1/L) × 100
Result4 = {(C4 × V) + [(C1 + C2 + C3 ) × VS]} × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 4.
Table 4
| Time Point (i) | Time (h) | Amount Dissolved (for Tablets that contain 100 mg of carbamazepine) (%) | Amount Dissolved (for Tablets that contain 200 or 400 mg of carbamazepine) (%) |
| 1 | 3 | 10-30 | 13-33 |
| 2 | 6 | 40-60 | 42-62 |
| 3 | 12 | 65-85 | 68-88 |
| 4 | 24 | NLT 80 | NLT 80 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 5: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 5.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL, deaerated
For Tablets labeled to contain 400 mg: Water; 1800 mL, deaerated
Apparatus 1: 10-mesh basket, 100 rpm
Use a suitable sinker1 for Tablets labeled to contain 100 or 200 mg. For Tablets with a release hole, orient the hole facing downward in the basket.
Times: 3, 6, 12, and 24 h
Standard solution
For Tablets labeled to contain 100 mg: 0.11 mg/mL of USP Carbamazepine RS prepared as follows. Transfer a suitable amount of USP
Carbamazepine RS to a suitable volumetric flask. Add methanol to 2.5% of the flask volume. Sonicate to dissolve. Dilute with Medium to volume.
For Tablets labeled to contain 200 or 400 mg: 0.22 mg/mL of USP Carbamazepine RS prepared as follows. Transfer a suitable amount of
USP Carbamazepine RS to a suitable volumetric flask. Add methanol to 5% of the flask volume. Sonicate to dissolve. Dilute with Medium to volume.
Sample solution: At the speci
ed time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of 0.45- μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 284 nm
Cell
For Tablets labeled to contain 100 mg: 0.2 cm
For Tablets labeled to contain 200 or 400 mg: 0.1 cm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Resulti = (Au/As ) × Cs
AU = absorbance from the Sample solution at time point i
As = absorbance from the Standard solution
Cs = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = {[C2 × (V − VS)] + (C1 × VS)} × (1/L) × 100
Result3 = ({C3 × [V − (2 × VS)]} + [(C2 + C1 ) × VS ]) × (1/L) × 100
Result4 = ({C4 × [V − (3 × VS)]} + [(C3 + C2 + C1 ) × VS]) × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point from the Medium (mL)
Tolerances: See Table 5.
Table 5
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 3 | 18-38 |
| 2 | 6 | 46-66 |
| 3 | 12 | 70-90 |
| 4 | 24 | NLT 80 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times speciFIed conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 6: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 6.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL, deaerated
For Tablets labeled to contain 400 mg: Water; 1800 mL, deaerated
Apparatus 2: 75 rpm
Times: 1, 3, 8, and 24 h
Solution A: Dilute 0.1 mL of phosphoric acid with water to 10 mL
Solution B: 1000 mL of water. Adjust with Solution A to a pH of 3.5.
Mobile phase: Methanol and Solution B (80:20)
Standard stock solution: 0.22 mg/mL of USP Carbamazepine RS prepared as follows. Transfer a suitable amount of USP Carbamazepine
RS to a suitable volumetric flask. Add methanol to 5% of the flask volume. Sonicate to dissolve. Dilute with Medium to volume.
Standard solution
For Tablets labeled to contain 100 mg: 0.11 mg/mL of USP Carbamazepine RS from the Standard stock solution in Medium
For Tablets labeled to contain 200 or 400 mg: 0.22 mg/mL of USP Carbamazepine RS from the Standard stock solution without dilution
Sample solution: At the specified time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of 0.45-μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained. Replace the portion removed from the solution under test with the same volume of Medium.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Flow rate: 1 mL/min
Injection volume: 5 μL
Run time: NLT 2 times the retention time of carbamazepine
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Resulti = (ru/rs ) × Cs
ru = peak response of carbamazepine from the Sample solution
rs = peak response of carbamazepine from the Standard solution
Cs = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2xV2) + (C1xVS) x (1/L) x 100
Result3 = {(C3 × V) + [(C1 + C2 ) × VS]} × (1/L) × 100
Result4 = {(C4 × V) + [(C1 + C2 + C3 ) × VS]} × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 6.
Table 6
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | 14-34 |
| 2 | 3 | 35-55 |
| 3 | 8 | 60-80 |
| 4 | 24 | NLT 80 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 7: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 7.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL, deaerated
For Tablets labeled to contain 400 mg: Water; 1800 mL, deaerated
Apparatus 1: 100 rpm with sinker. [Note—A suitable 8-mesh basket sinker with cover may be used.]
For Tablets with a release hole, orient the hole facing downward in the basket.
Times
For Tablets labeled to contain 100 mg: 2, 4, 6, 12, and 24 h
For Tablets labeled to contain 200 or 400 mg: 2, 4, 6, and 24 h
Mobile phase: Methanol, water, triethylamine, and trifluoroacetic acid (60: 40: 0.1: 0.1)
Standard solution: 0.22 mg/mL of USP Carbamazepine RS prepared as follows. Weigh a suitable amount of USP Carbamazepine RS in a suitable volumetric flask. Add methanol to 5% of the flask volume and sonicate to dissolve. Dilute with Medium to volume.
Sample solution: At the specified time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of 0.45-μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Flow rate: 1 mL/min
Injection volume: 10 μL
Run time: NLT 2 times the retention time of carbamazepine
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 5.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Resulti = (ru/rs ) × Cs
ru = peak response of carbamazepine from the Sample solution
rs = peak response of carbamazepine from the Standard solution
Cs= concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = {[C2 × (V − VS)] + (C1 × VS)} × (1/L) × 100
Result3 = ({C3 × [V − (2 × VS)]} + [(C2 + C1 ) × VS ]) × (1/L) × 100
Result4 = ({C4 × [V − (3 × VS)]} + [(C3 + C2 + C1 ) × VS]) × (1/L) × 100
Result5 = ({C5 × [V − (4 × Vs)]} + [(C4 + C3 + C2 + C1 ) × Vs]) × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
Vs = volume of the Sample solution withdrawn at each time point from the Medium (mL)
Tolerances: See Table 7 and Table 8.
Table 7
| Time Point (i) | Time (h) | Amount Dissolved (for Tablets that contain 100 mg of carbamazepine) (%) |
| 1 | 2 | NMT 20 |
| 2 | 4 | 20-40 |
| 3 | 6 | 40-60 |
| 4 | 12 | 65-85 |
| 5 | 24 | NLT 80 |
Table 8
| Time Point (i) | Time (h) | Amount Dissolved (for Tablets that contain 200 mg of carbamazepine) (%) | Amount Dissolved (for Tablets that contain 400 mg of carbamazepine) (%) |
| 1 | 2 | NMT 30 | NMT 20 |
| 2 | 4 | 35-55 | 25-45 |
| 3 | 6 | 55-75 | 45-65 |
| 4 | 24 | NLT 80 | NLT 80 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2.
Test 10: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 10.
Medium
For Tablets labeled to contain 100 or 200 mg: Water; 900 mL, deaerated, if necessary
For Tablets labeled to contain 400 mg: Water; 1800 mL, deaerated, if necessary
Apparatus 1: 10-mesh basket, 100 rpm
Use a suitable sinker.2
For Tablets with a release hole, orient the hole facing downward in the basket.
Times: 2, 4, 8, and 20 h
Solution A: Dilute 10 mL of phosphoric acid with water to 100 mL.
Buffer: Dissolve 1.36 g of potassium phosphate, monobasic in 1000 mL of water. Add 2 mL of triethylamine. Adjust with Solution A to a pH of 6.0.
Mobile phase: Acetonitrile, methanol, and Buffer (20:22:58)
Diluent: Methanol and water (80:20)
Standard stock solution: 1.12 mg/mL of USP Carbamazepine RS in Diluent. Sonicate to dissolve, if necessary.
Standard solution
For Tablets labeled to contain 100 mg: 0.112 mg/mL of USP Carbamazepine RS from the Standard stock solution in Medium
For Tablets labeled to contain 200 or 400 mg: 0.224 mg/mL of USP Carbamazepine RS from the Standard stock solution in Medium
Sample solution: At the specified time points, withdraw a suitable volume of the solution under test. Pass through a suitable filter of 0.45-μm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained. Replace the portion removed from the solution under test with the same volume of Medium.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 305 nm
Column: 4.6-mm × 15-cm; 5-μm packing L7
Column temperature: 50°
Flow rate: 1.5 mL/min
Injection volume: 5 μL
Run time: NLT 1.9 times the retention time of carbamazepine
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of carbamazepine (C15H12N2O) in the sample withdrawn from the vessel at each time point (i):
Result = (rS/rU) × CS
rU = peak response of carbamazepine from the Sample solution
rS = peak response of carbamazepine from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of carbamazepine (C15H12N2O) dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = [(C2xV2) + (C1xVS) x (1/L) x 100
Result3 = {(C3 × V) + [(C1 + C2 ) × VS]} × (1/L) × 100
Result4 = {(C4 × V) + [(C1 + C2 + C3 ) × VS]} × (1/L) × 100
Ci = concentration of carbamazepine in the portion of the sample withdrawn at time point i (mg/mL)
V = volume of Medium, 900 or 1800 mL
L = label claim (mg/Tablet)
Vs = volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 9.
Table 9
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 2 | NMT 20 |
| 2 | 4 | 35-55 |
| 3 | 8 | 65-85 |
| 4 | 20 | NLT 80 |
The percentages of the labeled amount of carbamazepine (C15H12N2O) dissolved at the times specified conform to Dissolution 〈711〉,
Acceptance Table 2. (RB 1-Jul-2024)
Uniformity of Dosage Units 〈905〉: Meet the requirements
5 IMPURITIES
Organic Impurities: Procedure 1
Mobile phase: Methanol, methylene chloride, and water (450:45:600)
System suitability solution: 60 μg/mL of phenytoin and 20 μg/mL of USP Carbamazepine RS in methanol
Standard solution: 4 μg/mL of USP Carbamazepine RS in methanol
Sample solution: Use Sample stock solution A from the Assay.
Chromatographic system and System suitability: Proceed as directed in the Assay.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU/rS) x (CS/CU ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of carbamazepine from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
CU = nominal concentration of carbamazepine in the Sample solution (mg/mL)
Acceptance criteria
Any individual unspecified degradation product: NMT 0.2%
Organic Impurities: Procedure 2
Mobile phase: Methanol, acetonitrile, and water (35:15:50)
System suitability solution: 12.5 μg/mL of iminostilbene and 5.0 μg/mL of USP Carbamazepine RS in methanol
Standard solution: 4 μg/mL of USP Carbamazepine RS in methanol
Sample solution: Use Sample stock solution A from the Assay.
Chromatographic system: Proceed as directed in the Assay.
System suitability
Sample: System suitability solution
[Note—The relative retention times for carbamazepine and iminostilbene are about 0.3 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 10.0 between carbamazepine and iminostilbene
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU/rS) x (CS/CU ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of carbamazepine from the Standard solution
CS = concentration of USP Carbamazepine RS in the Standard solution (mg/mL)
CU = nominal concentration of carbamazepine in the Sample solution (mg/mL)
Acceptance criteria
Any individual unspecified degradation product: NMT 0.2%
Total impurities: NMT 0.5% for all impurities from Procedure 1 and Procedure 2.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
Labeling: The labeling states the Dissolution test used only if Test 1 is not used.
USP Reference Standards 〈11〉
USP Carbamazepine RS


