Captopril
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
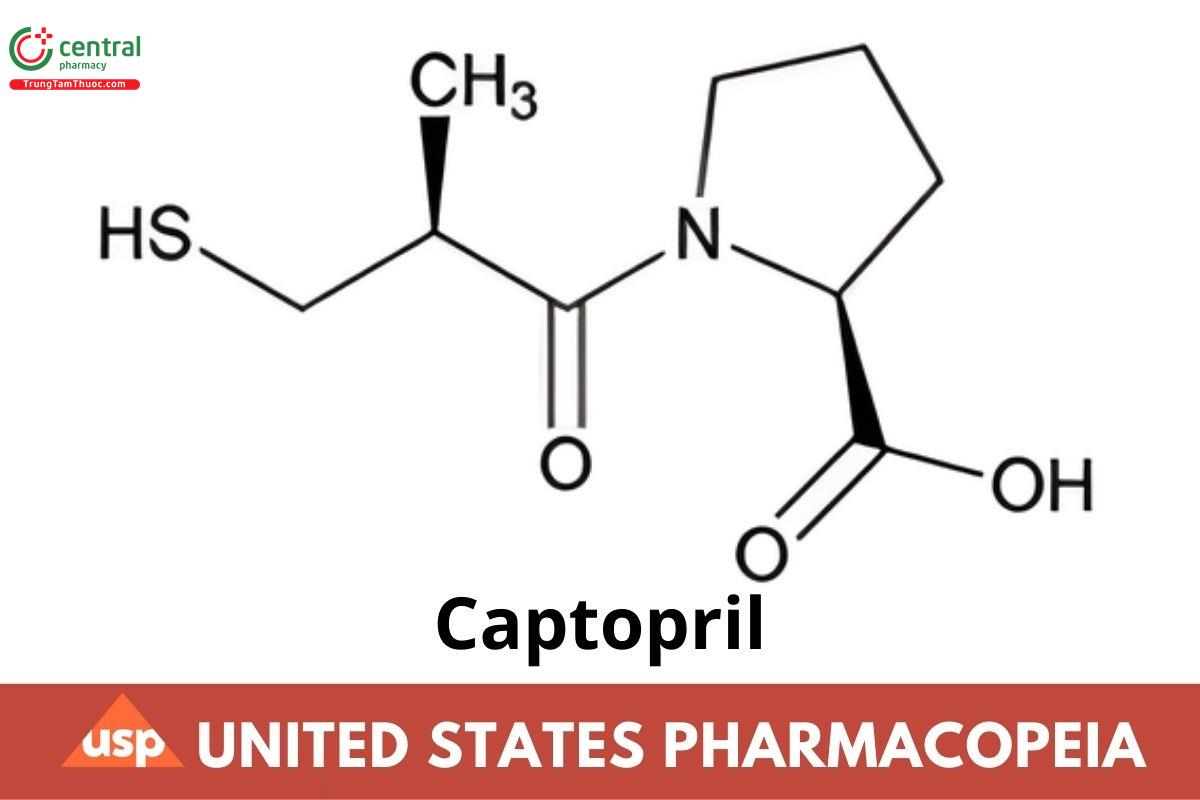
C9H15INO3S 217.29
L-Proline, 1-[(2S)-3-mercapto-2-methyl-1-oxopropyl]-;
1-[(2S)-3-Mercapto-2-methylpropionyl]-L-proline CAS RN: 62571-86-2; UNII: 9G64RSX1XD.
1 DEFINITION
Captopril contains NLT 97.5% and NMT 102.0% of captopril (C9H15INO3S), calculated on the dried basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
3 ASSAY
PROCEDURE
Sample solution: Dissolve about 300 mg of Captopril in 100 mL of water in a suitable glass-stoppered fiask. Add 10 mL of 3.6 N sulfuric acid, 1 g of potassium iodide, and 2 mL of starch TS.
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: Dissolve 3.567 g of potassium iodate, previously dried at 110° to constant weight, in water to make 1.0 L.
Endpoint detection: Visual
Analysis: Titrate with Titrant to a faint blue endpoint that persists for NLT 30 s. Perform a blank determination, and make any necessary correction. Each mL of Titrant is equivalent to 21.73 mg of captopril aptopril (C9H15INO3S).
Acceptance criteria: 97.5%-102.0% on the dried basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.2%
ORGANIC IMPURITIES
Use low-actinic glassware to prepare the Standard solution and Sample solution.
Mobile phase: 9-in-100 solution of tetrahydrofuran in methanol and 1-in-2000 solution of phosphoric acid (33:67)
System suitability stock solution: 0.1 mg/ml. each of USP Captopril RS, USP Captopril Disulfide RS, and 3-acetylthio-2-methylpropanoic acid in methanol
System suitability solution: 10 µg/mL each of USP Captopril RS, USP Captopril Disulfide RS, and 3-acetylthio-2-methylpropanoic acid in methanol from System suitability stock solution
Standard solution: 10 µg/mL of USP Captopril Disulfide RS in methanol
Sample solution: 2 mg/mL of Captopril in methanol. Use the solution promptly.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 3.9-mm x 30-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for captopril, 3-acetylthio-2-methylpropanoic acid, and captopril disulfide are 0.32, 0.42, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 3.0 between captopril and 3-acetylthio-2-methylpropanoic acid
Analysis
Samples: Standard solution and Sample solution
Compare the peak responses from the Sample solution, excluding those of the solvent, captopril, and captopril disulfide, with the main peak response from the Standard solution.
Calculate the percentage of captopril disulfide in the portion of Captopril taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of captopril disulfide from the Sample solution
rs = peak response of captopril disulfide from the Standard solution
CS = concentration of USP Captopril Disulfide RS in the Standard solution (μg/mL)
Cu = concentration of Captopril in the Sample solution (μg/mL)
Acceptance criteria: NMT 1.0% of captopril disulfide. The peak response of each impurity does not exceed 40% of the main peak response from the Standard solution (0.2%), and the sum of the impurity peak responses does not exceed the main peak response from the Standard solution (0.5%).
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 10 mg/mL in dehydrated alcohol
Acceptance criteria: −125° to −134°
Loss on Drying 〈731〉
Analysis: Dry a sample under vacuum at 60° for 3 h.
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Change to read:
USP Reference Standards 〈11〉
USP Captopril RS
USP Captopril Disulfide RS
(2′S)-[(2S,2′S)-3,3′-Disulfanediylbis(2-methylpropanoyl)]di-l-proline. (ERR 1-Dec-2021)
C18H28N2O6S2 432.55


