Capecitabine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
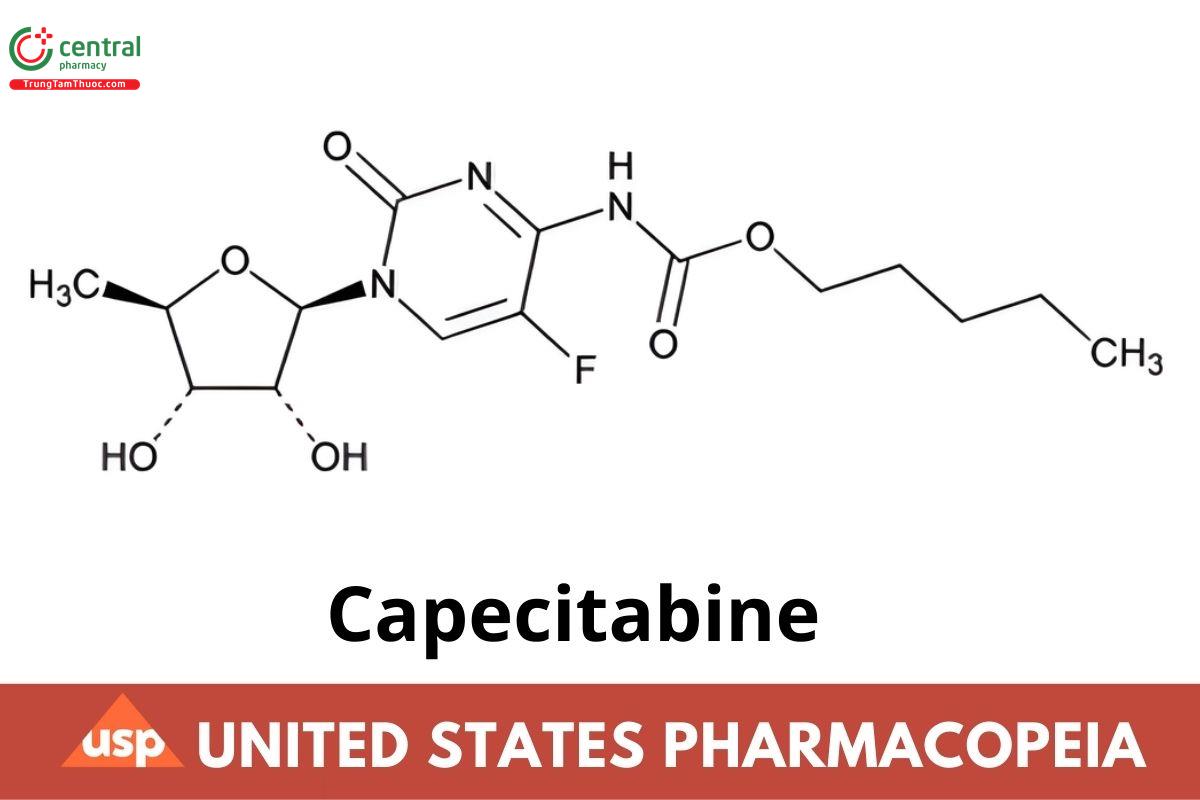
1 DEFINITION
Capecitabine Tablets contain NLT 93.0% and NMT 105.0% of the labeled amount of capecitabine (C15H22FN3O6).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
Analytical wave number: 1500–1760 cm−1
Sample: Grind 1 Tablet to a fine powder with a mortar and pestle. Mix 1 mg of this sample with 300 mg of potassium bromide.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Diluent: Methanol, acetonitrile, and water (7:1:12)
Solution A: 0.1% mixture of glacial acetic acid in water
Solution B: Methanol, acetonitrile, and Solution A (7:1:12)
Solution C: Methanol, acetonitrile, and Solution A (16:1:3)
Mobile phase: See the gradient table below.
| Time (min) | Solution B (%) | Solution C (%) |
| 0 | 100 | 0 |
| 5 | 100 | 0 |
| 20 | 49 | 51 |
| 30 | 49 | 51 |
| 31 | 100 | 0 |
| 40 | 100 | 0 |
[Note—The following solutions may be sonicated as necessary.]
System suitability solution: Includes 0.6 μg/mL of USP Capecitabine RS, 0.6 μg/mL of USP Capecitabine Related Compound A RS, 0.6 μg/mL of USP Capecitabine Related Compound B RS, and 0.6 μg/mL of USP Capecitabine Related Compound C RS in Diluent
Standard solution: 0.6 mg/mL of USP Capecitabine RS in Diluent
Sample solution: Equivalent to 0.6 mg/mL of capecitabine, from powdered Tablets (NLT 20), in Diluent.[Note—Pass through a PVDF membrane filter of 0.45-μm pore size, and use the filtrate.]
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 250 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Column temperature: 40°
Autosampler temperature: 5°
Flow rate: 1 mL/min
Injection size: 10 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—For the purpose of peak identification, the approximate relative retention times are given in Impurity Table 1. The relative retention times are measured with respect to capecitabine.]
Suitability requirements
Resolution: NLT 1.0 between capecitabine related compound A and capecitabine related compound B, System suitability solution
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C15H22FN3O6 in the portion of Tablets taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Capecitabine RS in the Standard solution (mg/mL)
CU = nominal concentration of capecitabine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
4.1 Organic Impurities
Procedure
Diluent, Solution A, Solution B, Solution C, Mobile phase, System suitability solution, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU/rS) x (CS/CU) × 100/F
r = peak response for each impurity from the Sample solution
r = peak response for capecitabine from the Standard solution
C = concentration of USP Capecitabine RS in the Standard solution (mg/mL)
C = nominal concentration of capecitabine in the Sample solution (mg/mL)
F = relative response factor for each impurity, from Impurity Table 1
4.2 Acceptance criteria
Individual impurities: See Impurity Table 1.
Total degradation products: NMT 1.5%
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Capecitabine related compound A | 0.18 | 1.05 | 0.3 |
| Capecitabine related compound B | 0.19 | 0.81 | 0.3 |
| 2',3'-Di-O-acetyl-5'-deoxy-5-fluorocytidine | 0.36 | 0.89 | 0.1 |
| 5'-Deoxy-5-fluoro-N4(2-methyl-1-butylxoxycarbonyl)cytidine + 5'-Deoxy-5-fluoro-N4-(3-methyl-1-butylxoxycarbonyl)cytidine | 0.95 | 1.01 | 0.5 |
| Capecitabine | 1.00 | 1.00 | — |
| [1-[5-Deoxy-3-O-(5-deoxy-β-D-ribofuranosyl)-β-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester | 1.06 | 1.00 | 0.3 |
| [1-[5-Deoxy-2-O-(5-deoxy-β-D-ribofuranosyl)-β-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester | 1.09 | 1.00 | 0.2 |
| Capecitabine related compound C | 1.11 | 0.91 | 0.3 |
| [1-[5-Deoxy-3-O-(5-deoxy-α-D-ribofuranosyl)-β-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester | 1.20 | 1.00 | 0.3 |
| 2',3'-Di-O-acetyl-5'-deoxy-5-fluoro-N4-(pentylxoxycarbonyl)cytidine | 1.37 | 0.85 | 0.1 |
| Individual unspecified impurity | — | 1.00 | 0.1 |
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation 〈781S〉: +96.0° to +100.0°
Sample solution: 10 mg/mL, on the anhydrous and solvent-free basis, in methanol, at 20°
Water Determination, Method Ic 〈921〉: NMT 0.3%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Capecitabine RS
USP Capecitabine Related Compound A RS
5′-Deoxy-5-fluorocytidine.
C9H12FN3O4 245.21
USP Capecitabine Related Compound B RS
5′-Deoxy-5-fluorouridine.
C9H11FN2O5 246.19
USP Capecitabine Related Compound C RS
2′,3′-O-Carbonyl-5′-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine.
C16H20FN3O7 385.34


