Candesartan Cilexetil
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
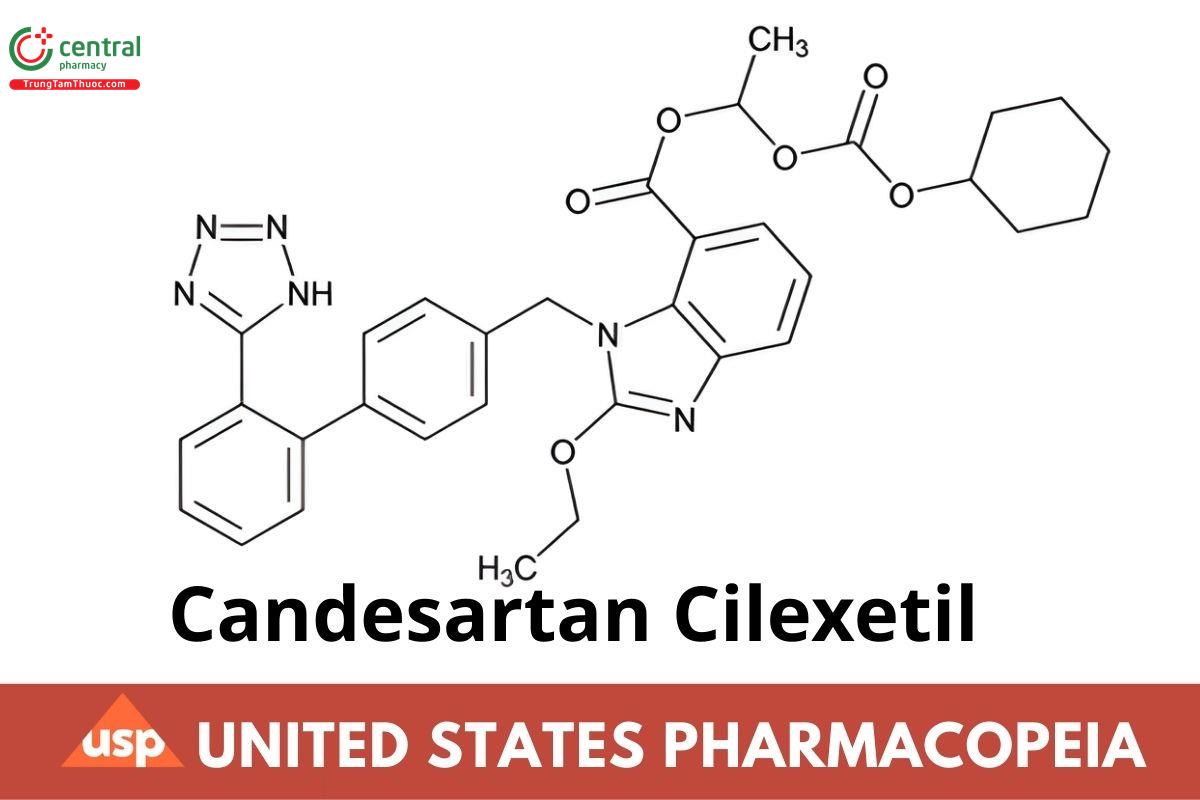
C33H34N6O6 610.67 (USP 1-Dec-2022)
1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, (±);
(±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester);
1-Cyclohexyloxycarbonyloxyethyl 2-ethoxy-1-{[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylate (USP 1-Dec-2022) CAS
RN®: 145040-37-5; UNII: R85M2X0D68.
Change to read:
1 DEFINITION
Candesartan cilexetil contains NLT 98.0% (USP 1-Dec-2022) and NMT 102.0% (USP 1-Dec-2022) of candesartan cilexetil (C33H34N6O6), calculated on anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or (USP 1-Dec-2022) 197K. If the spectra obtained show differences, proceed with the samples prepared as follows. Separately dissolve a quantity of USP Candesartan Cilexetil RS and Candesartan Cilexetil in alcohol. [Note—Heating the solution may be necessary for complete dissolution.] Cool the solution in an ice bath, filter the crystals, and dry at 105°.
Change to read:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP 1-Dec-2022)
3 ASSAY
Change to read:
Procedure
Solution A: Acetonitrile and water (57:43). To each liter, add 10 mL of glacial acetic acid.
Solution B: Acetonitrile and water (90:10). To each liter, add 10 mL of glacial acetic acid.
Mobile phase: See Table 1.
Table 1
| Time (Min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 3 | 100 | 0 |
| 33 | 0 | 100 |
| 33.1 | 100 | 0 |
Diluent: Acetonitrile and water (60:40)
Standard solution: 0.04 mg/mL of USP Candesartan Cilexetil RS in Diluent. Sonicate as necessary.
Sample solution: 0.04 mg/mL of Candesartan Cilexetil in Diluent. Sonicate as necessary.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm × 15-cm; 4-μm packing L1
Flow rate: 0.8 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of candesartan cilexetil (C33H34N6O6) in the portion of Candesartan Cilexetil taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of candesartan cilexetil from the Sample solution
rS = peak response of candesartan cilexetil from the Standard solution
CS = concentration of USP Candesartan Cilexetil RS in the Standard solution (mg/mL)
CU = concentration of Candesartan Cilexetil in the Sample solution (mg/mL) (USP 1-Dec-2022)
Acceptance criteria: 98.0%–102.0% (USP 1-Dec-2022) on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉
Sample: 1 g
Acceptance criteria: NMT 0.1%
Change to read:
Organic Impurities
Solution A, Solution B, Mobile phase, Diluent, and Chromatographic system: Proceed as directed in the Assay. (USP 1-Dec-2022)
System suitability solution: 0.4 mg/mL of USP Candesartan Cilexetil RS, and 0.4 μg/mL each of USP Candesartan Cilexetil Related
Compound A RS, USP Candesartan Cilexetil Related Compound B RS, USP Candesartan Cilexetil Related Compound D RS, USP Candesartan
Cilexetil Related Compound F RS, and USP Candesartan Cilexetil Related Compound G RS in Diluent (USP 1-Dec-2022)
Standard solution: 0.4 μg/mL (USP 1-Dec-2022) of USP Candesartan Cilexetil RS in Diluent
Sensitivity solution: 0.2 μg/mL of USP Candesartan Cilexetil RS in Diluent, from Standard solution (USP 1-Dec-2022)
Sample solution: 0.4 mg/mL of Candesartan Cilexetil in Diluent (USP 1-Dec-2022)
System suitability
(USP 1-Dec-2022)
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—See Table 2 for the relative retention times.] (USP 1-Dec-2022)
Suitability requirements
Resolution: NLT 4.0 between candesartan cilexetil related compound A and candesartan cilexetil related compound B; NLT 2.0 between
candesartan cilexetil and candesartan cilexetil related compound D, System suitability solution (USP 1-Dec-2022)
Tailing factor: NMT 1.5 for candesartan cilexetil, Standard solution (USP 1-Dec-2022)
Relative standard deviation: NMT 5.0% (USP 1-Dec-2022) for candesartan cilexetil, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution (USP 1-Dec-2022)
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of any specified and unspecified (USP 1-Dec-2022) impurity in the portion of Candesartan Cilexetil taken:
Result = (rU/rS) x (CS/CU) × (1/F) (USP 1-Dec-2022) × 100
rU = peak response of any specified or unspecified (USP 1-Dec-2022) impurity from the Sample solution
rS = peak response of candesartan cilexetil from the Standard solution
CS = concentration of USP Candesartan Cilexetil RS in the Standard solution (mg/mL)
CU = concentration of Candesartan Cilexetil in the Sample solution (mg/mL)
F = relative response factor (see Table 2) (USP 1-Dec-2022)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%. (USP 1-Dec-2022)
Table 2
| Name | Relative Retention Time | Relative Response Factor (USP 1-Dec-2022) | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Candesartan cilexetil related compound G | 0.19 | 1.2 | 0.2 (USP 1-Dec-2022) |
| Candesartan cilexetil related compound A | 0.35 | 1.1 (USP 1-Dec-2022) | 0.2 |
| Candesartan cilexetil related compound B | 0.47 | 0.93 (USP 1-Dec-2022) | 0.3 |
| Candesartan cilexetil | 1.0 | (USP 1-Dec-2022) | – |
| Candesartan cilexetil related compound D | 1.14 | 0.83 | 0.10 (USP 1-Dec-2022) |
| Candesartan cilexetil related compound F | 2.09 | 0.78 (USP 1-Dec-2022) | 0.2 |
| Trityl candesartan cilexetila | 3.62 | 0.40 | 0.15 (USP 1-Dec-2022) |
| Any unspecified impurity (USP 1-Dec-2022) | – | 1.0 (USP 1-Dec-2022) | 0.10 |
| Total impurities | – | –(USP 1-Dec-2022) | 0.6 |
a 1-Cyclohexyloxycarbonyloxyethyl 2-ethoxy-1-{[2'-(1-trityl-1H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylate.
5 SPECIFIC TESTS
Change to read:
Water Determination 〈921〉, Method I, Method Ia (USP 1-Dec-2022) : NMT 0.3%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
Change to read:
USP Reference Standards 〈11〉
USP Candesartan Cilexetil RS
USP Candesartan Cilexetil Related Compound A RS
Ethyl 2-ethoxy-1-{[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylate;
Also known as (Ethyl 1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-2-ethoxybenzimidazole-7-carboxylate).
C26H24N6O3 468.52
USP Candesartan Cilexetil Related Compound B RS
1-Cyclohexyloxycarbonyloxyethyl 1-{[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-2-oxo-2,3-dihydrobenzimidazole-7-carboxylate;
Also known as (1-{[(Cyclohexyloxy)carbonyl]oxy}ethyl 3-({2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl}methyl)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylate).
C31H30N6O6 582.62
USP Candesartan Cilexetil Related Compound D RS
1-{[(Cyclohexyloxy)carbonyl]oxy}ethyl 1-{[2’-(2-ethyl-2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-2-oxo-2,3-dihydrobenzimidazole-7-carboxylate;
Also known as 1-{[(Cyclohexyloxy)carbonyl]oxy}ethyl 3-({2'-(2-ethyl-2H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl}methyl)-2-oxo-2,3-dihydro-1H-benzimidazole-4-carboxylate).
C33H34N6O6 610.67
USP Candesartan Cilexetil Related Compound F RS
1-Cyclohexyloxycarbonyloxyethyl 2-ethoxy-1-{[2’-(2-ethyl-2H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylate;
Also known as (1-(Cyclohexyloxycarbonyloxy)ethyl 2-ethoxy-1-{[2'-(2-ethyl-2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate).
C35H38N6O6 638.73
USP Candesartan Cilexetil Related Compound G RS
2-Ethoxy-1-{[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylic acid;
Also known as 1-{[2’-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl}-2-ethoxybenzimidazole-7-carboxylic acid.
C24H20N6O3 440.46 (USP 1-Dec-2022)


