Calcium Pantothenate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
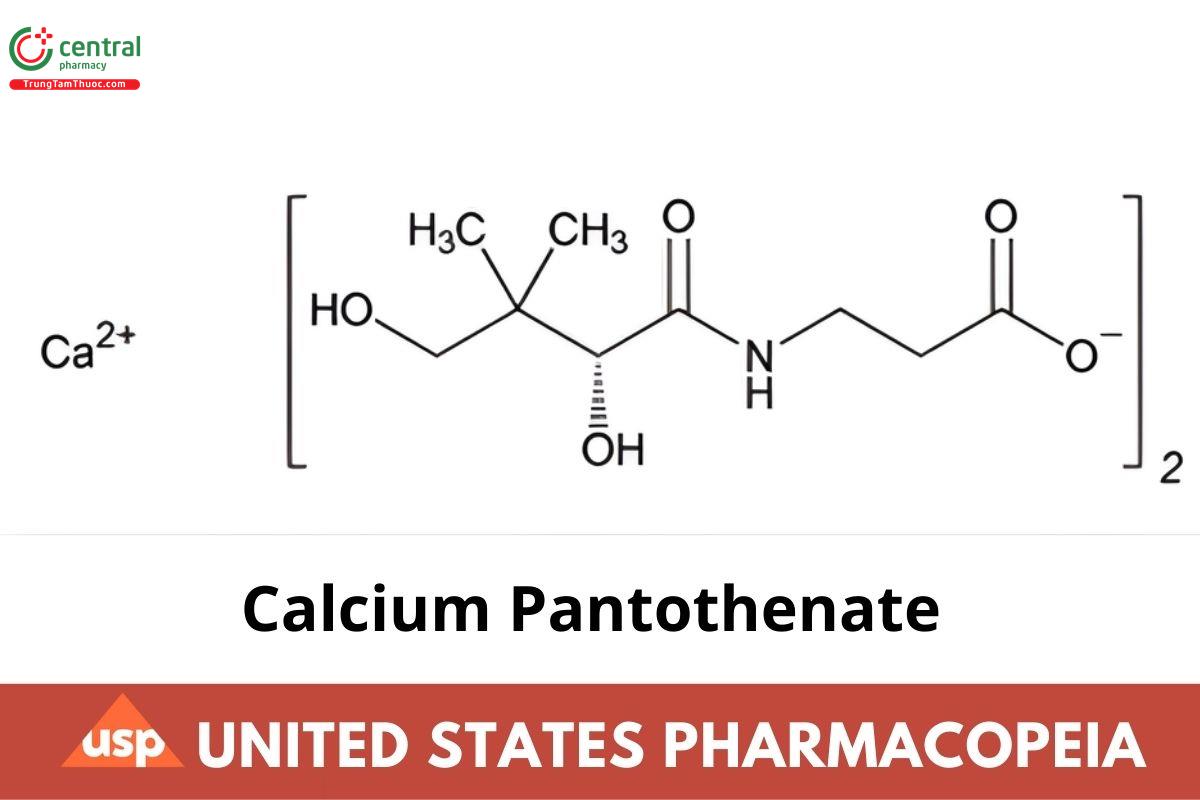
C18H32CaN2O10 476.53
β-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-, calcium salt (2:1), (R)-;
Calcium d-pantothenate (1:2) CAS RN®: 137-08-6; UNII: 568ET80C3D.
1 DEFINITION
Calcium Pantothenate is the calcium salt of the dextrorotatory isomer of pantothenic acid. It contains NLT 98.0% and NMT 102.0% of calcium
pantothenate (C18H32CaN2O10), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Calcium
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
C. Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 50 mg/mL in water
Acceptance criteria: +25.0° to +27.5°
3 ASSAY
Change to read:
Procedure
Mobile phase: Dissolve 1.56 g of monobasic sodium phosphate in 900 mL of water, and adjust the pH to 2.5 with concentrated ortho-phosphoric acid. Add 10 mL of acetonitrile and dilute with water to 1000 mL.
System suitability solution: 0.04 mg/mL of USP Calcium Pantothenate RS and 0.1 mg/mL of USP Pantolactone RS in water
Standard solution: 0.5 mg/mL of USP Calcium Pantothenate RS in water
Sample solution: 0.5 mg/mL of Calcium Pantothenate in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 3.0-mm × 15-cm; 3.5-μm packing L1
Column temperature: 35°
Flow rate: 1.2 mL/min
Injection volume: 5 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for pantolactone and pantothenic acid are 0.8 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 3.0 between pantolactone and pantothenic acid peaks, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of calcium pantothenate (C18H32CaN2O10) in the portion of Calcium Pantothenate taken:
Result = (rU /rS) × (CS /CU ) x 100
rU = peak area of pantothenic acid from the Sample solution
rS = peak area of pantothenic acid from the Standard solution
CS = concentration of USP Calcium Pantothenate RS in the Standard solution (mg/mL)
CU = concentration of Calcium Pantothenate in the Sample solution (mg/mL)
(USP 1-Dec-2023)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 OTHER COMPONENTS
Content of Calcium
Sample: 800 mg of Calcium Pantothenate
Blank: 150 mL of water containing 2 mL of 3 N hydrochloric acid
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.05 M edetate disodium VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 150 mL of water containing 2 mL of 3 N hydrochloric acid. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and titrate with Titrant to a distinct blue endpoint. Perform the blank determination.
Calculate the percentage of calcium (Ca) in the Sample taken:
Result = {[(VS − VB ) × M × F]/W} × 100
VS = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
M = actual molarity of the Titrant (mmol/mL)
F = equivalency factor, 40.08 mg/mmol
W = Sample weight (mg)
Acceptance criteria: 8.2%–8.6% on the dried basis
Add the following:
5 IMPURITIES
Beta Alanine and Other Aminocarboxylic Acid Impurities
Sample: 8.000 g of Calcium Pantothenate
Blank: 100 mL of water and 25 mL of formaldehyde solution
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide VS
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 40 mL of water and dilute with the same solvent to 100 mL. Add 25 mL of formaldehyde solution and titrate with Titrant. Perform the blank determination.
Calculate the percentage of β-alanine in the Sample taken:
Result = [(VS − VB ) × N × F/W] × 100
VS= Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
N = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 89.09 mg/mEq
W = Sample weight (mg)
Acceptance criteria: NMT 0.5%
Other Related Substances
Mobile phase and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 0.004 mg/mL of USP Calcium Pantothenate RS, 0.04 mg/mL of USP Sodium d-Pantoate RS, and 0.02 mg/mL of USP
Pantolactone RS in water
Sample solution: 4 mg/mL of Calcium Pantothenate in water
System suitability
Sample: Standard solution
[Note—The relative retention times for pantoic acid, pantolactone, and pantothenic acid are 0.5, 0.8, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 3.0 between pantolactone and pantothenic acid peaks
Relative standard deviation: NMT 5.0% for pantoic acid, pantolactone, and pantothenic acid peaks
Analysis
Samples: Standard solution and Sample solution
Run time: 30 min
Calculate the percentage of pantoic acid in the portion of Calcium Pantothenate taken:
Result = (rU /rS) × (CS /CU )× (Mt1 /Mt2) × 100
rU = peak area of pantoic acid from the Sample solution
rS = peak area of pantoic acid from the Standard solution
CS = concentration of USP Sodium d-Pantoate RS in the Standard solution (mg/mL)
CU = concentration of Calcium Pantothenate in the Sample solution (mg/mL)
Mt1 = molecular weight of pantoic acid, 148.16
Mt2 = molecular weight of sodium pantoate, 170.14
Calculate the percentage of pantolactone in the portion of Calcium Pantothenate taken:
Result = (rU /rS) × (CS /CU ) x 100
rU = peak area of pantolactone from the Sample solution
rS = peak area of pantolactone from the Standard solution
CS = concentration of USP Pantolactone RS in the Standard solution (mg/mL)
CU = concentration of Calcium Pantothenate in the Sample solution (mg/mL)
Disregarding any peak due to beta alanine and other aminocarboxylic acid impurities with relative retention times below 0.2, calculate the
percentage of β-alanyl pantothenamide and any unspecified impurity in the portion of Calcium Pantothenate taken using the concentration of calcium pantothenate in the Standard solution:
Result = (rU /rS) × (CS /CU ) x 100
rU = peak response of β-alanyl pantothenamide or any unspecified impurity from the Sample solution
rS = peak response of pantothenic acid from the Standard solution
CS = concentration of USP Calcium Pantothenate RS in the Standard solution (mg/mL)
CU = concentration of Calcium Pantothenate in the Sample solution (mg/mL)
Acceptance criteria: See Table 1. The reporting threshold is 0.05%.
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Pantoic acid | 0.5 | 1.0 |
| Pantolactone | 0.8 | 0.5 |
| Pantothenic acid | 1.0 | — |
| β-Alanyl pantothenamide | 1.7 | 0.25 |
6 SPECIFIC TESTS
6.1 Alkalinity
Sample: 1.0 g
Analysis: Dissolve the Sample in 15 mL of carbon dioxide-free water in a small ask. As soon as the solution is complete, add 1.0 mL of 0.10N hydrochloric acid, and then add 0.05 mL of phenolphthalein TS.
Acceptance criteria: No pink color is produced within 5 s.
6.2 Loss on Drying 〈731〉
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 5.0%
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Calcium Pantothenate RS
USP Pantolactone RS
USP Sodium d-Pantoate RS


