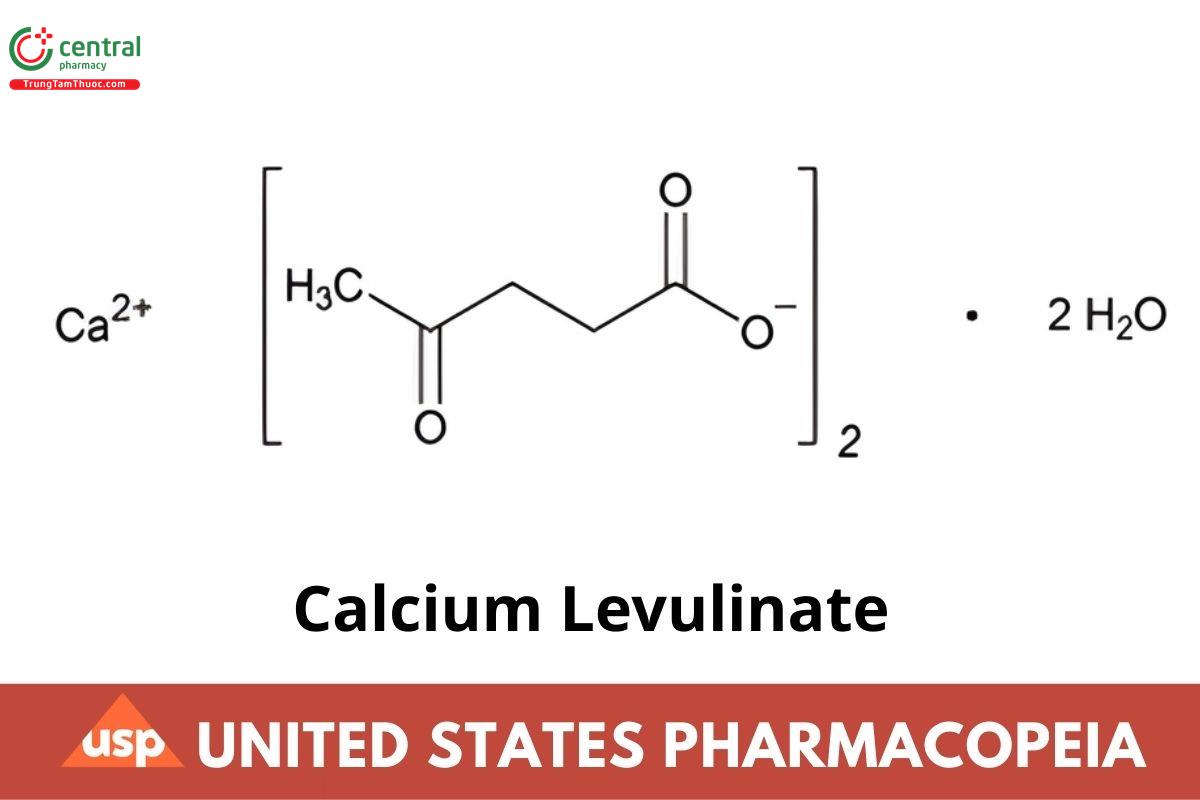
Calcium Levulinate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
C10H14CaO6.2H₂O 306.32
C10H14CaO6 270.30
Pentanoic acid, 4-oxo-, calcium salt (2:1), dihydrate;
Calcium levulinate (1:2) dihydrate CAS RN: 5743-49-7; UNII: T6133S0781. Anhydrous CAS RN®: 591-64-0; UNII: LLQ966USIL.
1 DEFINITION
Calcium Levulinate contains NLT 97.5% and NMT 100.5% of calcium levulinate (C10H14CaO6), calculated on the dried basis.
2 IDENTIFICATION
A. IDENTIFICATION TESTS-GENERAL, Calcium (191): A 100 mg/mL solution meets the requirements.
B.Sample solution: 0.5 g in 5 mL of water
Analysis: To the Sample solution add 5 mL of 1 N sodium hydroxide, and filter. To the filtrate add 5 mL of iodine TS.
Acceptance criteria: A precipitate of iodoform is produced.
3 ASSAY
PROCEDURE
Sample: 600 mg of Calcium Levulinate
Blank: 150 mL of water containing 2 mL of 3 N hydrochloric acid
Titrimetric system (See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.05 M edetate disodium VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 150 mL of water containing 2 mL of 3 N hydrochloric acid. While stirring with a magnetic stirrer, add 30 mL of Titrant from the titration buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration to a blue endpoint. Perform the Blank determination.
Calculate the percentage of calcium levulinate (C10H14CaO6) in the Sample taken:
Result = {[(VS-VB) x Mx F/W) x 100
Vs = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
M = Titrant molarity (mM/mL)
F = equivalency factor, 270.3 mg/mM
W = Sample weight (mg)
Acceptance criteria: 97.5%-100.5% on the dried basis
4 IMPURITIES
4.1 CHLORIDE AND SULFATE, Chloride (221).
Standard: 1.0 mL of 0.020 N hydrochloric acid
Sample: 1.0 g
Acceptance criteria: NMT 0.07%
4.2 CHLORIDE AND SULFATE, Sulfate (221).
Standard: 1.0 mL of 0.020 N sulfuric acid
Sample: 2.0 g
Acceptance criteria: NMT 0.05%
4.3 REDUCING SUGARS
Sample: 0.50 g
Analysis: Dissolve the Sample in 10 mL of water, add 2 mL of 3 N hydrochloric acid, boil for about 2 min, and cool. Add 5 mL of sodium carbonate TS, allow to stand for 5 min, dilute with water to 20 mL, and filter. Add 5 mL of the clear filtrate to 2 mL of alkaline cupric tartrate TS, and boil for 1 min.
Acceptance criteria: No red precipitate is formed immediately.
5 SPECIFIC TESTS
MELTING RANGE OR TEMPERATURE, Class I(741): 119°-125°
PH (791).
Sample solution: 100 mg/mL
Acceptance criteria: 7.0-8.5
LOSS ON DRYING (731): Dry a sample at a pressure not exceeding 5 mm of mercury at 60° for 5 h: it loses 10.5%-12.0% of its weight.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers.


