Calcium Gluconate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
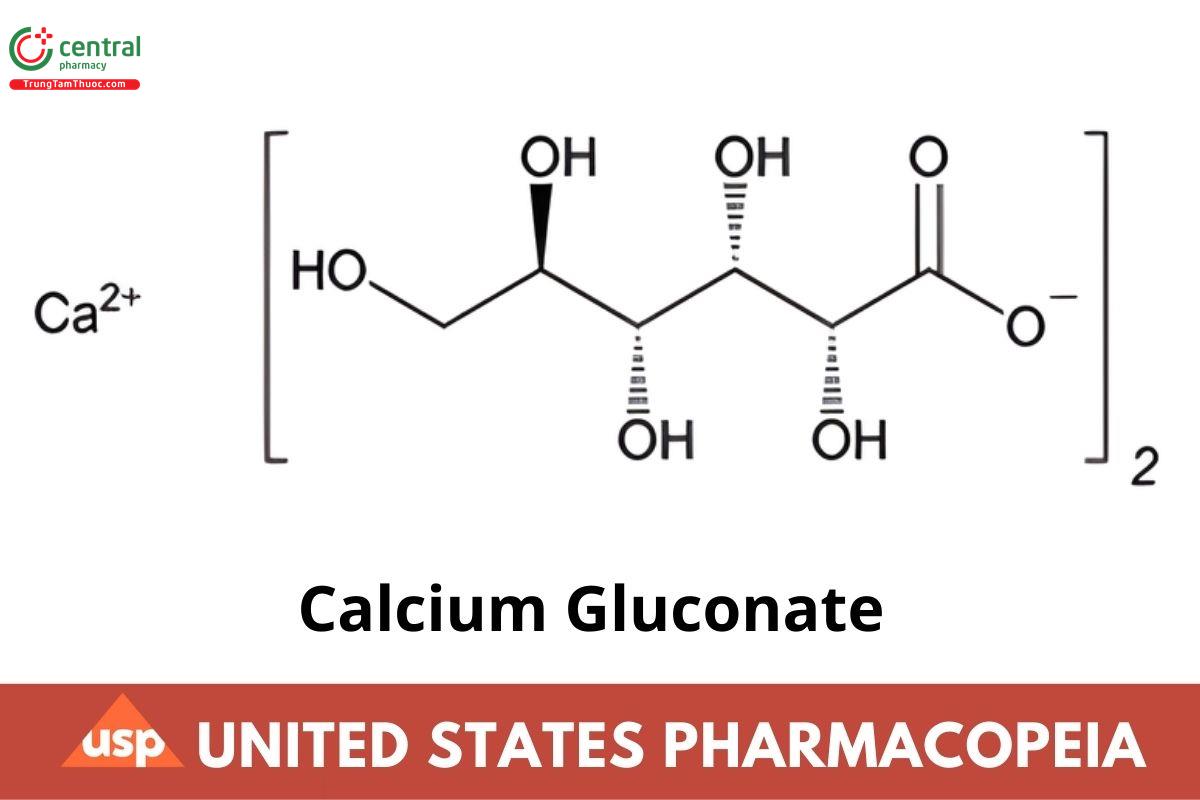
C12H22CaO14 430.37
C12H22CaO14.H2O 448.39
D-Gluconic acid, calcium salt (2:1);
Calcium D-gluconate (1:2) CAS RN®: 299-28-5; UNII: SQE6VB453K.
Monohydrate CAS RN®: 66905-23-5; UNII: CZNOMI5R31.
1 DEFINITION
Calcium Gluconate is anhydrous or contains one molecule of water of hydration. The anhydrous form contains NLT 98.0% and NMT 102.0% of calcium gluconate (C12H22CaO14), calculated on the dried basis. The monohydrate form contains NLT 99.0% and NMT 101.0% of calcium gluconate monohydrate (C12H22CaO14.H2O) where labeled as intended for use in preparing injectable dosage forms, and NLT 98.5% and NMT 102.0% of calcium gluconate monohydrate (C12H22CaO14.H2O) where labeled as not intended for use in preparing injectable dosage forms.
2 IDENTIFICATION
2.1 A. IDENTIFICATION TESTS GENERAL (191), Calcium
Sample solution: 20 mg/mL
Acceptance criteria: Meets the requirements
2.2 B. INFRARED ABSORPTION
Standard preparation: Prepare USP Calcium Gluconate Anhydrous RS or USP Calcium Gluconate Monohydrate RS in the form of a potassium bromide pellet.
Sample preparation: Prepare calcium gluconate anhydrous or calcium gluconate monohydrate in the form of a potassium bromide pellet.
Analysis
Samples: Standard preparation and Sample preparation
Record the spectra over the range from about 2.6 to 15 μm (3800-650 cm-1).
Acceptance criteria: The spectrum of Calcium Gluconate labeled as anhydrous exhibits the differential maxima at the wavenumbers in Table 1 that are consistent with those of the spectrum of USP Calcium Gluconate Anhydrous RS. The spectrum of Calcium Gluconate labeled as monohydrate exhibits the differential maxima at the wavenumbers in Table 1 that are consistent with those of the spectrum of USP Calcium Gluconate Monohydrate RS.
| Spectral Region | Wavenumber (cm⁻¹) of Characteristic Absorptions for Anhydrous | Wavenumber (cm⁻¹) of Characteristic Absorptions for Monohydrate |
|---|---|---|
| O–H stretching | No sharp band at about 3485, only broad absorption | At about 3485 (medium sharp over broad absorption) |
| C=O stretching | 1618 (strong) | 1595 (strong) |
| Fingerprint | 1329 (medium) | Not observed |
| Not observed | 1305 (medium) | |
| 1263–1250 (strong, two fused bands) | Not observed | |
| Not observed | 1236 (medium) | |
| 1007 (medium) | Not observed | |
| Not observed | 1045 (medium) | |
| 948 (duplet, medium) | Not observed | |
| Not observed | 972 (weak) | |
| 865 (weak) | Not observed | |
| Not observed | 878 (weak) | |
| 766 (medium) | Not observed |
3 ASSAY
PROCEDURE
Sample: 800 mg of calcium gluconate anhydrous or calcium gluconate monohydrate
Blank: 150 mL of water containing 2 mL of 3 N hydrochloric acid
Titrimetric system (See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.05 M edetate disodium VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 150 mL of water containing 2 mL of 3 N hydrochloric acid. While stirring, add 30 mL of Titrant from the titration buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration to a blue endpoint.
Perform the Blank determination. Calculate the percentage of calcium gluconate (C12H22CaO14) or calcium gluconate monohydrate (C12H22CaO14.H2O) in the Sample taken:
Result (anhydrous form) = {[(VS-VB) x MxF]/W} x 100
Result (monohydrate form) = {[(VS-VB)×MxF]/W}x (Mr2/Mr1) × 100
Vs= Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
M = Titrant molarity (mmol/mL)
F = equivalency factor, 430.4 mg/mmol
W = Sample weight (mg)
Mr2 = molecular weight of calcium gluconate monohydrate, 448.4
Mr1 = molecular weight of calcium gluconate anhydrous, 430.4
Acceptance criteria
Anhydrous: 98.0%-102.0% on the dried basis
Monohydrate: 99.0%-101.0% where labeled as intended for use in preparing injectable dosage forms; 98.5%-102.0% where labeled as not intended for use in preparing injectable dosage forms
4 IMPURITIES
Change to read:
ARSENIC (211), Procedures, Procedure 1 (CN 1-JUN-2023)
Test preparation: Dissolve 1.0 g in a mixture of 10 mL of hydrochloric acid and 20 mL of water, and dilute with water to 55 mL..
Analysis: Proceed as directed in the chapter, except to omit the addition of 20 mL of 7 N sulfuric acid.
Acceptance criteria: NMT 3 ppm
CHLORIDE AND SULFATE (221), Chloride: A 1.0-g portion shows no more chloride than corresponds to 0.07 mL of 0.020 N hydrochloric acid
(0.005%). Where it is labeled as not intended for use in the preparation of injectable dosage forms, a 1.0-g portion shows no more chloride than corresponds to 1 mL of 0.020 N hydrochloric acid (0.07%).
CHLORIDE AND SULFATE (221), Sulfate: A 2.0-g portion dissolved in boiling water shows no more sulfate than corresponds to 0.1 ml. of 0.020 N sulfuric acid (0.005%). Where it is labeled as not intended for use in the preparation of injectable dosage forms, a 2.0-g portion dissolved in boiling water shows no more sulfate than corresponds to 1 mL of 0.020 N sulfuric acid (0.05%).
LIMIT OF IRON
[NOTE-Calcium Gluconate labeled as not intended for use in the preparation of injectable dosage forms is exempt from this requirement.]
Internal standard solution: 2 µg/mL of yttrium in 10% nitric acid, prepared from commercially prepared yttrium standard suitable for ICP-OES. [NOTE-The yttrium concentration can be varied to optimize the analysis.)
Standard solutions: 0.05, 0.1, 0.2, and 0.4 µg/mL of iron in 10% nitric acid, prepared from commercially prepared iron standard suitable for ICP-OES
Sample solution: 40 mg/mL of Calcium Gluconate in 10% nitric acid
Blank solution: 10% Nitric acid solution
Instrumental conditions
(See Plasma Spectrochemistry (730).)
Mode: Inductively coupled plasma-optical emission spectroscopy (ICP-OES)
Emission wavelengths: 239.562 nm or optimized wavelength for iron, 371.029 nm or optimized wavelength for yttrium
System suitability
Samples: Internal standard solution, Standard solutions, and Blank solution
Instrument performance must be verified to conform to the manufacturer's specifications for resolution and sensitivity. Before analyzing samples, the instrument must pass a suitable performance check. Generate the calibration curve using the Blank solution and Standard solutions as follows. Scan the Internal standard solution while running the Blank solution to measure the intensity of the yttrium emission. Hold this value constant throughout the remainder of the test. Separately scan the Blank solution; Standard solutions of 0.05, 0.1, 0.2, and 0.4 µg/mL of iron; and Internal standard solution. NOTE-Add the internal standard solution via an in-line mixing chamber. Normalize the yttrium intensity to the value of the Internal standard solution. Apply this normalization factor to the iron intensity, which is then referred to as the corrected iron intensity. Construct a calibration curve by plotting the corrected iron intensity versus the known concentrations, in µg/mL, of the iron: the linear regression coefficient is NLT 0.999.
Analysis
Sample: Sample solution
Similarly, analyze the Sample solution on the ICP. Plot the intensity of the emission of the Sample solution on the calibration curve. Obtain the concentration of iron, C, in µg/mL, in the Sample solution through the calibration curve.
Calculate the content, in µg/g (ppm), of iron in the portion of Calcium Gluconate taken:
Result = (CxV)/W
C = concentration of iron in the Sample solution obtained from the calibration curve (µg/mL)
V = volume of the Sample solution (mL)
W = weight of Calcium Gluconate taken to prepare the Sample solution (g)
Acceptance criteria: NMT 5 ppm
LIMIT OF MAGNESIUM AND ALKALI METALS
NOTE-Calcium Gluconate labeled as not intended for use in preparing injectable dosage forms is exempt from this requirement.
Sample: 1.0 g
Analysis: Dissolve the Sample completely in 100 mL of boiling water. Add 10 mL of ammonium chloride TS, 1 mL of ammonium hydroxide, and 50 ml of hot (maintained at 70-80°) ammonium oxalate TS. Allow to stand for 4 h, dilute with water to 200 ml, and filter. Evaporate 100 ml. of the filtrate to dryness, and ignite to constant weight.
Acceptance criteria: NMT 0.4%; the weight of the residue does not exceed 2 mg.
LIMIT OF PHOSPHATE
[NOTE-Calcium Gluconate labeled as not intended for use in the preparation of injectable dosage forms is exempt from this requirement.]
Standard stock solution 1: 0.716 mg/ml of monobasic potassium phosphate
Standard stock solution 2: Dilute 1.0 mL of Standard stock solution 1 with water to 100 mL
Standard solution: Dilute 2.0 mL of Standard stock solution 2 with water to 100 mL.
Sample stock solution: To 10.0 g of Calcium Gluconate add 90 ml. of hot water (70-80"), and heat to boiling, with swirling, for 10 s to obtain a clear solution.
Sample solution: Dilute 1 mL of the hot Sample stock solution with water to 100 mL.
Analysis
Samples: Standard solution and Sample solution
To the Standard solution and Sample solution add 4 ml of sulfomolybdic acid TS, and mix. To both solutions add 0.1 mL of a freshly prepared mixture of 3 N hydrochloric acid and stronger acid stannous chloride TS (10:1), and mix.
Acceptance criteria: NMT 0.01%; after 10 min any color in the Sample solution is not more intense than that in the Standard solution.
LIMIT OF OXALATE
[NOTE-Calcium Gluconate labeled as not intended for use in the preparation of injectable dosage forms is exempt from this requirement.] [NOTE-Use deionized water where water is indicated.]
Solution A: 0.0125 M sulfuric acid in water
Solution B: Dilute 1 mL of hydrochloric acid with water to 1200 mL.
Mobile phase: 0.0017 M sodium bicarbonate and 0.0018 M sodium carbonate in water.
Standard solution: 1.5 µg/mL of sodium oxalate in Solution B
Sample solution: 20 mg/mL of Calcium Gluconate in Solution B. Sonicate if necessary.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: lon chromatography
Detector: Conductance
Columns
Guard: 4-mm x 5-cm; 15-um packing L12
Analytical: 4-mm x 25-cm; 15-um packing L12
Anion suppressor: The micromembrane anion suppressor column is connected in series with the guard and analytical columns. The anion suppressor column is equipped with a micromembrane that separates Mobile phase from Solution A flowing countercurrent to Mobile phase at a rate of about 7 mL/min. [NOTE-Condition the system for about 15 min with Mobile phase at a flow rate of 2 mL/min.]
Flow rate: 2 mL/min
Injection volume: 50 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 2500 theoretical plates.
Tailing factor: NMT 1.2
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of oxalate in the portion of Calcium Gluconate taken:
Result = (rU/rS) x (CS/CU) × (Mr1/Mr2)×Fx100
rU = peak response of oxalate from the Sample solution
rS = peak response of oxalate from the Standard solution
CS = concentration of sodium oxalate in the Standard solution (µg/mL)
CU = concentration of Calcium Gluconate in the Sample solution (mg/mL)
Mr1 = molecular weight of oxalate, 88.03
Mr2= molecular weight of sodium oxalate, 134.00
F = conversion factor, 0.001 mg/µg
Acceptance criteria: NMT 0.01%
REDUCING SUBSTANCES
Sample: 1.0 g of Calcium Gluconate
Blank: 20 ml of water
Titrimetric system
(See Titrimetry (541).)
Mode: Residual titration
Titrant: 0.1 N iodine VS
Back titrant: 0.1 N sodium thiosulfate VS
Endpoint detection: Visual
Analysis: Transfer the Sample to a 250-mL conical flask, dissolve in 20 mL of hot water, cool, and add 25 mL of alkaline cupric citrate TS. Cover the flask, boil gently for 5 min, accurately timed, and cool rapidly to room temperature. Add 25 mL of 0.6 N acetic acid, 10.0 mL of Titrant, and 10 mL. of 3 N hydrochloric acid. Titrate with the Back titrant, adding 3 mL of starch TS as the endpoint is approached. Perform the Blank determination. Calculate the percentage of reducing substances (as dextrose) in the Sample taken:
Result = {[(VB-VS)xNxF]/W}×100
VB = Back titrant volume consumed by the Blank (mL)
VS = Back titrant volume consumed by the Sample (mL)
N = Back titrant normality (mEq/mL)
F = equivalency factor, 27 mg/mEq
W = Sample weight (mg)
Acceptance criteria: NMT 1.0%
5 SPECIFIC TESTS
LOSS ON DRYING (731).
Analysis: Dry at 105° for 16 h.
Acceptance criteria
Anhydrous: NMT 3.0%
Monohydrate: NMT 1.0%, where labeled as intended for use in preparing injectable dosage forms; NMT 2.0%, where labeled as not intended for use in preparing injectable dosage forms
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers.
LABELING: Label it to indicate whether it is anhydrous or monohydrate. Where the quantity of calcium gluconate is indicated in the labeling of any solution containing Calcium Gluconate, this shall be understood to be in terms of anhydrous calcium gluconate (C12H22CaO14). Calcium Gluconate intended for use in preparing injectable dosage forms is so labeled. Calcium Gluconate not intended for use in preparing injectable dosage forms is so labeled; in addition, it may be labeled also as intended for use in preparing oral dosage forms.
USP REFERENCE STANDARDS (11)
USP Calcium Gluconate Anhydrous RS
USP Calcium Gluconate Monohydrate RS


