Calcium Gluceptate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
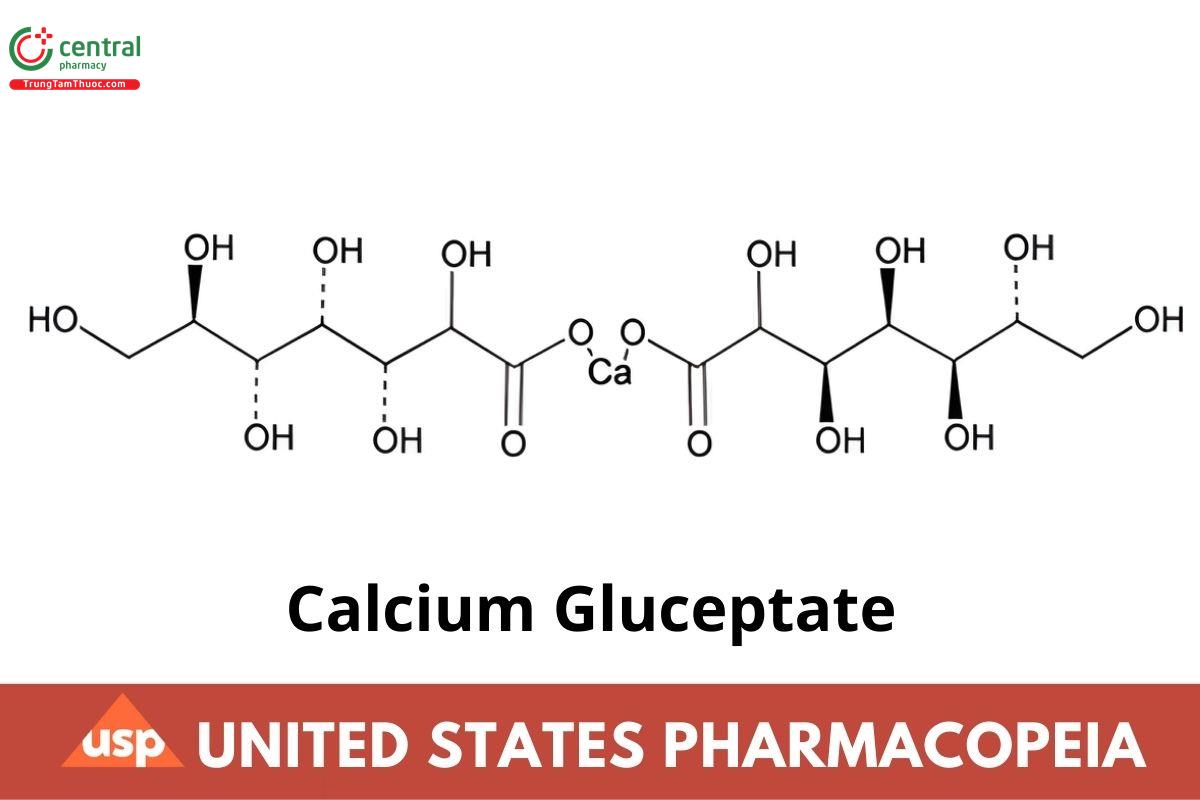
C14H26CaO16(anhydrous). 490.42
Glucoheptonic acid, calcium salt (2:1);
Calcium glucoheptonate (1:2) CAS RN: 29039-00-7; UNII: L11651398J.
1 DEFINITION
Calcium Gluceptate is anhydrous or contains varying amounts of water of hydration. It consists of the calcium salt of the alpha epimer of glucoheptonic acid or of a mixture of the alpha and beta epimers of glucoheptonic acid. It contains NLT 95.0% and NMT 102.0% of calcium gluceptate (C14H26CaO16), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. IDENTIFICATION TESTS-GENERAL, Calcium (191): A 20-mg/mL solution meets the requirements.
3 ASSAY
PROCEDURE
Sample: 800 mg of Calcium Gluceptate
Blank: 150 mL of water containing 2 mL of 3 N hydrochloric acid
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.05 M edetate disodium VS
Endpoint detection : Visual
Analysis: Dissolve the Sample with 150 mL of water containing 2 mL of 3 N hydrochloric acid. While stirring, preferably with a magnetic stirrer, add 25 mL of Titrant from the titration buret. Add 15 mL of 1 N sodium hydroxide and 300mg of hydroxy naphthol blue, and continue the titration to a blue endpoint. Perform the Blank determination.
Calculate the percentage of calcium gluceptate (C14H26CaO16) in the Sample taken:
Result = {[(VS-VB) x MxF]/W} x 100
VS = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
M = actual molarity of the Titrant (mM/mL)
F = equivalency factor, 490.4 mg/mM
W = Sample weight (mg)
Acceptance criteria: 95.0%-102.0% on the dried basis
4 IMPURITIES
4.1 CHLORIDE AND SULFATE, Chloride(221)
Standard: 1.0 mL of 0.020 N hydrochloric acid
Sample: 1.0 g
Acceptance criteria: NMT 0.07%
4.2 CHLORIDE AND SULFATE, Sulfate (221)
Standard: 1.0 mL of 0.020 N sulfuric acid
Sample: 2.0 g
Acceptance criteria: NMT 0.05%
Reducing Sugars
Sample: 0.50 g
Analysis: Dissolve the Sample in 10 mL of hot water, add 2 mL of 3 N hydrochloric acid, boil for about 2 min, and cool. Add 5 mL of sodium carbonate TS, allow to stand for 5 min, dilute with water to 20 mL, and filter. Add 5 mL of the clear filtrate to 2 mL of alkaline cupric tartrate TS, and boil for 1 min.
Acceptance criteria: No red precipitate is formed immediately.
5 SPECIFIC TESTS
5.1 pH 〈791〉
Sample solution: 100 mg/mL
Acceptance criteria: 6.0–8.0
5.2 Loss on Drying 〈731〉
(See Thermal Analysis 〈891〉.)
[Note—The quantity taken for the determination may be adjusted, if necessary, for instrument sensitivity. Weight loss occurring at temperatures above about 160°, indicative of decomposition, is not to be interpreted as Loss on Drying.]
Sample: 10–25 mg
Analysis: Determine the percentage of volatile substances by thermogravimetric analysis on an appropriately calibrated instrument. Heat the specimen under test at a rate of 5°/min in an atmosphere of nitrogen, at a flow rate of 40 mL/min. Record the thermogram to 150°.
Acceptance criteria: See Table 1.
Table 1
| Form | Loss on Drying (%) |
|---|---|
| Anhydrous | NMT 1.0 |
| 2H2O (dihydrate) | NMT 6.9 |
| 3 1/2 H2O | NMT 11.4 |
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
Labeling: Label to indicate whether hydrous or anhydrous; if hydrous, label to indicate also the degree of hydration.
USP Reference Standards 〈11〉
USP Calcium Gluceptate RS


