Calcium Citrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
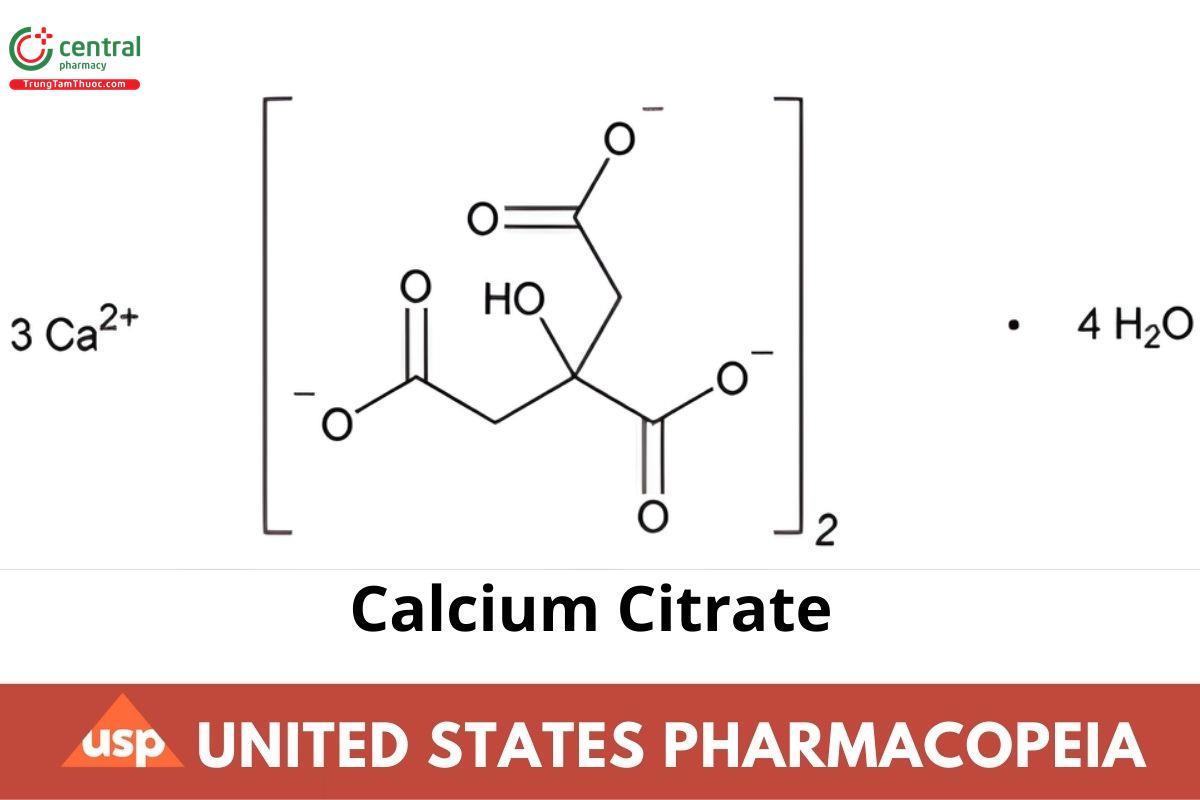
C12H10C3014.4H2O 570.49
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, calcium salt (2:3), tetrahydrate;
Calcium citrate (3:2), tetrahydrate CAS RN: 5785-44-4; UNII: MLM29U2X85.
1 DEFINITION
Calcium Citrate contains NLT 98.0% and NMT 102.0% of calcium citrate [Ca3(C6H5O7)2], calculated on the dried basis.
2 IDENTIFICATION
A.Sample: 0.5 g
Analysis: Dissolve the Sample in a mixture of 10 mL of water and 2.5 mL of 2 N nitric acid. Add 1 mL of mercuric sulfate TS, heat to boiling, and add 1 mL of potassium permanganate TS.
Acceptance criteria: A white precipitate is formed.
B. The retention time of the calcium peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Use water with a resistivity of NLT 18 megohm-cm to prepare the solutions.
Mobile phase: 34 mM methanesulfonic acid in water. [NOTE-It is recommended to use a suitable cation trapping technique to ensure that the Mobile phase is sufficiently free of cationic impurities.]
System suitability stock solution: 4 mg/mL of USP Calcium Citrate RS and 0.5 mg/mL of USP Magnesium Carbonate RS prepared as follows. Transfer an appropriate amount of USP Calcium Citrate RS and USP Magnesium Carbonate RS to a suitable volumetric flask. Add 6 N hydrochloric acid to about 10% of the final volume and dissolve. Dilute with water to volume.
System suitability solution: 0.08 mg/mL of USP Calcium Citrate RS and 0.01 mg/mL of USP Magnesium Carbonate RS in water from System suitability stock solution
Standard stock solution: 4 mg/mL of USP Calcium Citrate RS prepared as follows. Transfer an appropriate portion of USP Calcium Citrate RS to a suitable volumetric flask. Add 6 N hydrochloric acid to about 10% of the final volume and dissolve. Dilute with water to volume.
Standard solution: 0.08 mg/mL of USP Calcium Citrate RS in water from Standard stock solution
Sample stock solution: 4 mg/mL of Calcium Citrate prepared as directed for the Standard stock solution
Sample solution: 0.08 mg/mL of Calcium Citrate in water from the Sample stock solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Conductivity with suppression
Columns
Guard: 5.0-mm x 5-cm; 5.5-µm packing 184
Analytical: 5.0-mm x 25-cm; 5.5-µm packing L84
Column temperature: 50°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
Run time: NLT 2 times the retention time of calcium
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for the magnesium and calcium ions are 0.76 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between magnesium and calcium, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of calcium citrate Ca3(C6H5O7)2 in the portion of Calcium Citrate taken:
Result = (rU /rS) × (CS /CU ) x 100
rU = peak response of calcium from the Sample solution
rS = peak response of calcium from the Standard solution
CS = concentration of USP Calcium Citrate RS in the Standard solution (mg/mL)
CU = concentration of Calcium Citrate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%on the dried basis
4 IMPURITIES
Change to read:
Arsenic 〈211〉, Procedures, Procedure 1 (CN 1-Jun-2023)
Sample: 1 g
Analysis: Dissolve the Sample in 5 mL of 3 N hydrochloric acid, and dilute with water to 35 mL.
Acceptance criteria: NMT 3 ppm
Change to read:
Lead 〈251〉, Procedures, Procedure (CN 1-Jun-2023)
Standard solution: 5 mL of Diluted standard lead solution [5 μg of lead (Pb)]
Sample solution: Dissolve 0.5 g of Calcium Citrate in 20 mL of 3 N hydrochloric acid. Evaporate this solution to 10 mL on a steam bath, dilute with water to 20 mL, and cool.
Acceptance criteria: NMT 10 ppm
Limit of Fluoride
Use water with a resistivity of NLT 18 megohm-cm to prepare the solutions. Prepare and store all solutions in plastic containers.
Solution A: 50 mM potassium hydroxide in water
Solution B: Water
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 10 | 90 |
| 10 | 10 | 90 |
| 15 | 100 | 0 |
| 25 | 100 | 0 |
| 30 | 10 | 90 |
| 45 | 10 | 90 |
[Note—Alternatively, Mobile phase can be generated electrolytically using an automatic eluant generator. It is recommended to use suitable anion trapping techniques to ensure the Mobile phase is free of all anionic impurities.]
Diluent: 0.03 N hydrochloric acid and 0.03 N potassium hydroxide in water
System suitability solution: 0.13 μg/mL of USP Sodium Fluoride RS and 0.08 μg/mL of USP Sodium Acetate RS in water
Standard stock solution: 0.2 mg/mL of USP Sodium Fluoride RS prepared as follows. Transfer an appropriate amount of USP Sodium
Fluoride RS to a suitable volumetric ask. Add water to about 20% of the final volume, followed by 6 N hydrochloric acid to 0.5% of the final volume. Add 6 N potassium hydroxide to 0.5% of the final volume. Dilute with water to volume.
Standard solution: 0.13 μg/mL of USP Sodium Fluoride RS in Diluent from Standard stock solution
Sample solution: 2 mg/mL of Calcium Citrate prepared as directed in Standard stock solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Conductivity with suppression
Columns
Guard: 4.0-mm × 5-cm; 13-μm packing L120. [Note—Alternatively, a 4.0-mm × 0.5-cm column that contains 4.6-μm packing L91 may be used.]
Analytical: 4.0-mm × 25-cm; 7.5-μm packing L113. [Note—Alternatively, a 4.0-mm × 25-cm column that contains 4.6-μm packing L91 may be used.]
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for the fluoride and acetate peaks are 1.0 and 1.2, respectively.]
Suitability requirements
Resolution: NLT 1.5 between the
uoride and acetate peaks, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 20, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of fluoride in the portion of Calcium Citrate taken:
Result = (rU /rS ) × (CS /CU ) × (A r/Mr ) × 100
rU = peak response of fluoride from the Sample solution
rS = peak response of fluoride from the Standard solution
CS = concentration of USP Sodium Fluoride RS in the Standard solution (μg/mL)
CU = concentration of Calcium Citrate in the Sample solution (μg/mL)
Ar = atomic weight of fluoride, 19.00
Mr = molecular weight of sodium fluoride, 41.99
Acceptance criteria: NMT 0.003%
Limit of Acid-Insoluble Substances
Sample solution: Dissolve 5 g of Calcium Citrate by heating with 16.7% (v/v) hydrochloric acid for 30 min.
Analysis: Filter, wash, and dry at 105° for 2 h the residue so obtained.
Acceptance criteria: NMT 10 mg (0.2%)
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry at 150° for 4 h.
Acceptance criteria: 10.0%–13.3%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Calcium Citrate RS
USP Magnesium Carbonate RS
USP Sodium Acetate RS
USP Sodium Fluoride RS


