Calcium Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
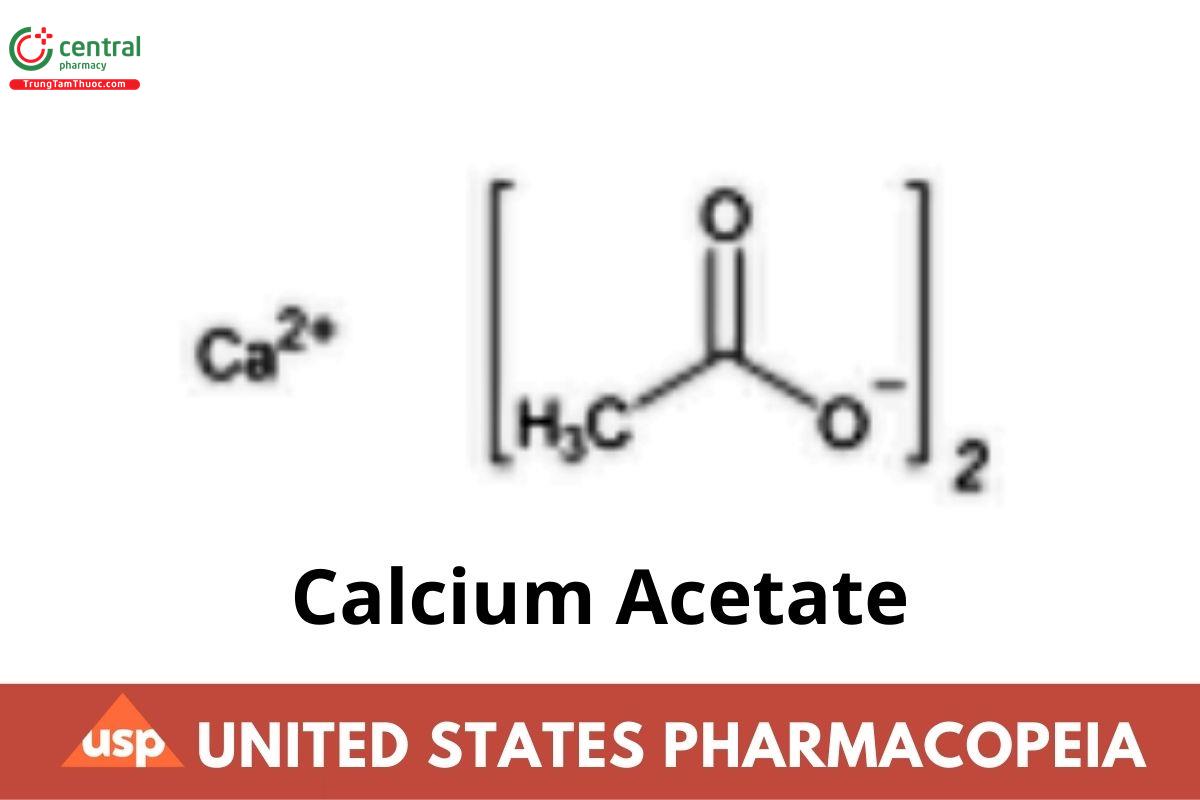
C4H6CaO4158.17
Acetic acid, calcium salt;
Calcium acetate CAS RN®: 62-54-4; UNII: Y882YXF34X.
1 DEFINITION
Calcium Acetate contains NLT 99.0% and NMT 100.5% of calcium acetate (C4H6CaO4), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Identification Tests—General, Calcium〈191〉andAcetate〈191〉
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Sample: 300 mg
Analysis: Dissolve the Sample in 150 mL of water containing 2 mL of 3 N hydrochloric acid. While stirring, preferably with a magnetic stirrer, add about 30 mL of 0.05 M edetate disodium VS from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration to a blue endpoint. Each mL of 0.05 M edetate disodium is equivalent to 7.909 mg of calcium acetate (C4H6CaO4).
Acceptance criteria: 99.0%–100.5% on the anhydrous basis
4 IMPURITIES
Change to read:
Arsenic 〈211〉, Procedures, Procedure 1 : NMT 3 ppm
Chloride and Sulfate, Chloride〈221〉
Standard: 0.70 mL of 0.020 N hydrochloric acid
Sample: 1.0 g
Acceptance criteria: 0.05%
Chloride and Sulfate, Sulfate〈221〉
Standard: 0.15 mL of 0.020 N sulfuric acid
Sample: 0.25 g
Acceptance criteria: 0.06%
Change to read:
Lead 〈251〉, Procedures, Procedure 1 : NMT 10 ppm
Change to read:
Limit of Aluminum
[Note—Use where it is labeled as intended for parenteral use or for use in hemodialysis or peritoneal dialysis.]
Buffer: Dissolve 50 g of ammonium acetate in 150 mL of water, adjust with glacial acetic acid to a pH of 6.0, and dilute with water to 250 mL. Aluminum standard solution: 1.0 µg/mL of aluminum. Prepare as directed for Standard Preparations in Aluminum 〈206〉, Procedures, Procedure 1 .
Standard solution: Prepare a solution containing 2.0 mL of Aluminum standard solution, 5 mL of Buffer, and 48 mL of water, and extract this solution with successive portions of 10, 10, and 5 mL of 0.5% 8-hydroxyquinoline in chloroform. Combine the chloroform extracts in a 50- mL volumetric flask. Dilute the combined extracts with chloroform to volume.
Sample solution: Dissolve 1.0 g of Calcium Acetate in 50 mL of water, and add 5 mL of Buffer. Extract this solution with successive portions of 10, 10, and 5 mL of 0.5% 8-hydroxyquinoline in chloroform. Combine the chloroform extracts in a 50-mL volumetric flask. Dilute the combined extracts with chloroform to volume.
Blank solution: Prepare a solution containing 50 mL of water and 5 mL of Buffer. Extract this solution with successive portions of 10, 10, and 5 mL of 0.5% 8-hydroxyquinoline in chloroform. Combine the chloroform extracts in a 50-mL volumetric flask. Dilute the combined extracts with chloroform to volume.
Instrumental conditions
(See Fluorescence Spectroscopy 〈853〉.)
Mode: Fluorescence
Excitation wavelength: 392 nm
Emission wavelength: 518 nm
Analysis
Samples: Standard solution, Sample solution, and Blank solution
Use the Blank solution to zero the instrument.
Acceptance criteria: 2 ppm; the fluorescence of the Sample solution is NMT that of the Standard solution.
Limit of Barium
[Note—Use where it is labeled as intended for use in hemodialysis or peritoneal dialysis.]
Barium chloride solution: 500 µg/mL of barium in water from anhydrous barium chloride
Buffer: Ammonium sulfate solution (1 in 10)
Standard solution: To a tube add 1 g of ammonium acetate, 2 mL of 1 N hydrochloric acid, 3.0 mL of Barium chloride solution, and sufficient water to bring the volume to 40 mL.
Sample stock solution: 250 mg/mL of Calcium Acetate and 25 mg/mL of ammonium acetate in 1 N hydrochloric acid. The pH of this solution is 4.5–5.5. Filter, and cover the solution.
Sample solutions: To four separate tubes add 1.0, 1.5, 2.0, and 2.5 mL of Barium chloride solution. To each tube add a sufficient volume of the Sample stock solution to bring the volume to 40 mL.
Analysis: To the Sample solutions and the Standard solution add, with brisk stirring, 3.0 mL of Buffer, and allow to stand for 20 min.
Acceptance criteria: The Sample solutions containing 1.0 and 1.5 mL of Barium chloride solution remain clear or are only faintly turbid. The Sample solution containing 2.0 mL of Barium chloride solution is not more turbid than the Standard solution.
Limit of Fluoride
[Note—Prepare and store all solutions in plastic containers.]
Buffer: 294 mg/mL of sodium citrate dihydrate in water
Standard stock solution: 1.11 mg/mL of USP Sodium Fluoride RS in water
Standard solution: Combine 20.0 mL of Standard stock solution with 50.0 mL of Buffer, and dilute with water to 100.0 mL. Equivalent to 100 µg/mL of Fluoride
Sample solution: Transfer 2.0 g of Calcium Acetate to a beaker containing a plastic-coated stirring bar. Add 20.0 mL of water and 2.0 mL of hydrochloric acid, and stir until dissolved. Add 50.0 mL of Buffer and sufficient water to make 100 mL.
Electrode system: Use a fluoride-specic, ion-indicating electrode and a silver–silver chloride reference electrode connected to a pH meter capable of measuring potentials with a minimum reproducibility of ±0.2 mV (see pH 〈791〉).
Analysis
Samples: Standard solution and Sample solution
Transfer 50.0 mL of Buffer and 2.0 mL of hydrochloric acid to a beaker, and add water to make 100 mL. Add a plastic-coated stirring bar, insert the electrodes into the solution, stir for 15 min, and read the potential, in mV. Continue stirring, and at 5-min intervals add 100, 100, 300, and 500 µL of the Standard solution, reading the potential 5 min after each addition. Plot the logarithms of the cumulative fluoride ion concentrations (0.1, 0.2, 0.5, and 1.0 µg/mL) versus potential, in mV.
Rinse and dry the electrodes, insert them into the Sample solution, stir for 5 min, and read the potential, in mV. From the measured potential and the standard response line determine the concentration, C, in µg/mL, of fluoride ion in the Sample solution. Calculate the amount of fluoride (ppm) in the sample taken by multiplying C by 50.
Acceptance criteria: 50 ppm
Limit of Magnesium
[Note—Use where it is labeled as intended for use in hemodialysis or peritoneal dialysis. The Standard solution and the Sample solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.] Standard stock solution: 1000 µg/mL of magnesium in 1 N nitric acid from magnesium oxide
Standard solution: 5.0 µg/mL of magnesium from the Standard stock solution
Sample solution: 2 mg/mL of Calcium Acetate
Linearity solution A: Dilute 20.0 mL of the Sample solution with water to 25.0 mL (0 µg/mL of magnesium).
Linearity solution B: Dilute 2.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (0.4 µg/mL of magnesium).
Linearity solution C: Dilute 4.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (0.8 µg/mL of magnesium).
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 285.2 nm
Flame: Air–acetylene
Lamp: Magnesium hollow-cathode
Blank: Water
Analysis
Samples: Linearity solutions A, B, and C
Plot the absorbances of the Linearity solutions versus their content of magnesium (0, 0.4, and 0.8 µg/mL), draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg/mL, of magnesium in the Sample solution.
Calculate the percentage of magnesium in the sample by multiplying this value by 0.0625.
Acceptance criteria: NMT 0.05%
Limit of Nitrate
Sample solution: 100 mg/mL of Calcium Acetate in water
Analysis: To 10 mL of the Sample solution add 5 mg of sodium chloride, 0.05 mL of indigo carmine TS, and, with stirring, 10 mL of nitrogen free sulfuric acid.
Acceptance criteria: The blue color persists for NLT 10 min.
Limit of Potassium
[Note—Use where it is labeled as intended for use in hemodialysis or peritoneal dialysis. The Standard solution and Sample solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.] Standard stock solution: 23.84 mg/mL of potassium chloride, using potassium chloride previously dried at 105° for 2 h, equivalent to 12.5 mg/mL of potassium
Standard solution: 31.25 µg/mL of potassium from the Standard stock solution
Sample solution: 12.5 mg/mL of Calcium Acetate
Linearity solution A: Dilute 20.0 mL of the Sample solution with water to 25.0 mL (0 µg/mL of potassium).
Linearity solution B: Dilute 2.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (2.5 µg/mL of potassium).
Linearity solution C: Dilute 4.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (5.0 µg/mL of potassium).
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 766.7 nm
Lamp: Potassium hollow-cathode
Flame: Air–acetylene
Blank: Water
Analysis
Samples: Linearity solutions A, B, and C
Plot the absorbances of the Linearity solutions versus their content of potassium (0, 2.5, and 5.0 µg/mL), draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg/mL, of potassium in the Sample solution.
Calculate the percentage of potassium in the sample by multiplying this value by 0.01.
Acceptance criteria: NMT 0.05%
Limit of Sodium
[Note—Use where it is labeled as intended for use in hemodialysis or peritoneal dialysis. The Standard solution and the Sample solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.] Standard stock solution: 25.42 mg/mL of sodium chloride, using sodium chloride previously dried at 105° for 2 h, equivalent to 10.0 mg/mL of sodium
Standard solution: 250 µg/mL of sodium from the Standard stock solution
Sample solution: 10 mg/mL of Calcium Acetate
Linearity solution A: Dilute 20.0 mL of the Sample solution with water to 25.0 mL (0 µg/mL of sodium).
Linearity solution B: Dilute 2.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (20 µg/mL of sodium). Linearity solution C: Dilute 4.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (40 µg/mL of sodium). Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 589.0 nm
Lamp: Sodium hollow-cathode
Flame: Air–acetylene
Blank: Water
Analysis
Samples: Linearity solutions A, B, and C
Plot the absorbances of the Linearity solutions versus their content of sodium (0, 20, and 40 µg/mL), draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg/mL, of sodium in the Sample solution.
Calculate the percentage of sodium in the sample by multiplying this value by 0.0125.
Acceptance criteria: NMT 0.5%
Limit of Strontium
[Note—Use where it is labeled as intended for use in hemodialysis or peritoneal dialysis. The Standard solution and Sample solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.] Standard stock solution: 2.45 mg/mL of strontium acetate in water, equivalent to 1000 µg/mL of strontium
Standard solution: 50.0 µg/mL of strontium from the Standard stock solution
Sample solution: 20 mg/mL of Calcium Acetate
Linearity solution A: Dilute 20.0 mL of the Sample solution with water to 25.0 mL (0 µg/mL of strontium).
Linearity solution B: Dilute 2.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (4 µg/mL of strontium). Linearity solution C: Dilute 4.0 mL of the Standard solution and 20.0 mL of the Sample solution with water to 25.0 mL (8 µg/mL of strontium). Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: 460.7 nm
Lamp: Strontium hollow-cathode
Flame: Nitrous oxide–acetylene
Blank: Water
Analysis
Samples: Linearity solutions A, B, and C
Plot the absorbances of the Linearity solutions versus their content of strontium (0, 4, and 8 µg/mL), draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg/mL, of strontium in the Sample solution.
Calculate the percentage of strontium in the sample by multiplying this value by 0.00625.
Acceptance criteria: NMT 0.05%
Readily Oxidizable Substances
Sample solution: 20 mg/mL of Calcium Acetate in boiling water
Analysis: Add a few glass beads to 100 mL of the Sample solution, 6 mL of 10 N sulfuric acid, and 0.3 mL of 1 N potassium permanganate. Mix, boil gently for 5 min, and allow the precipitate to settle.
Acceptance criteria: The pink color in the supernatant is not completely discharged.
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 50 mg/mL
Acceptance criteria: 6.3–9.6
Water Determination, Method I〈921〉
Sample: 0.100 g
Analysis: Proceed as directed in the chapter, adding 2 mL of glacial acetic acid to the titration vessel in addition to the methanol. Acceptance criteria: NMT 7.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Where Calcium Acetate is intended for use in hemodialysis or peritoneal dialysis, it is so labeled.
USP Reference Standards 〈11〉
USP Sodium Fluoride RS


