Calcitriol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
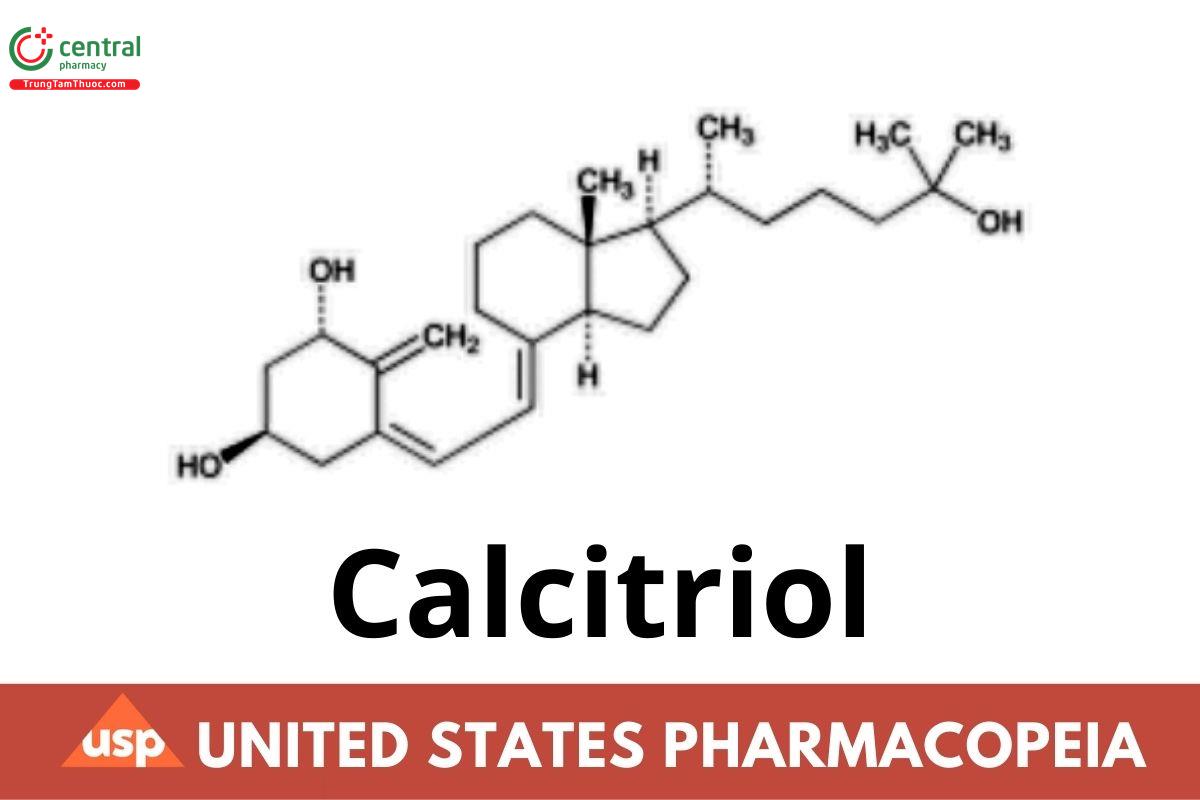
C27H44O3 416.64
C27H44O3.H2O 434.65
9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1α,3β,5Z,7E)-;
(5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1α,3β,25-triol CAS RN®: 32222-06-3. Monohydrate CAS RN®: 77326-95-5
1 DEFINITION
Calcitriol is anhydrous or contains 1 molecule of hydration. The anhydrous form contains NLT 97.0% and NMT 103.0% of calcitriol (C27H44O3),
calculated on the solvent-free basis. The monohydrate form contains NLT 97.0% and NMT 103.0% of calcitriol (C27H44O3), calculated on the 27
anhydrous basis.
[Caution—Care should be taken to prevent inhaling particles of calcitriol, and exposing the skin to it.]
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Carry out the procedure as rapidly as possible, and protect all solutions containing calcitriol from light.
Buffer: 1.0 mg/mL of tris(hydroxymethyl)aminomethane in water, adjusted with phosphoric acid to a pH of 7.0–7.5 before nal dilution Mobile phase: Acetonitrile and Buffer (55:45)
Standard solution: 0.1 mg/mL of USP Calcitriol RS prepared as follows. Transfer an appropriate amount of USP Calcitriol RS to a suitable volumetric flask, dissolve in acetonitrile, using 55% of the nal volume, then dilute with Buffer to volume.
System suitability solution: Heat 2.0 mL of the Standard solution at 80° for 30 min.
Sample solution: 0.1 mg/mL of Calcitriol prepared as follows. Transfer an appropriate amount of Calcitriol to a suitable volumetric flask, dissolve in acetonitrile, using 55% of the nal volume, then dilute with Buffer to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 25-cm; 5-µm packing L7
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 50 µL
Run time: NLT 2 times the retention time of calcitriol
System suitability
Samples: Standard solution andSystem suitability solution
[Note—The relative retention times for pre-calcitriol and calcitriol are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 3.5 between the pre-calcitriol and calcitriol peaks, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of calcitriol (C27H44O3) in the portion of Calcitriol taken:
Result = (rU/rS) × (CS/CU) × 100
rU = sum of the peak responses of calcitriol and pre-calcitriol from the Sample solution
rS = sum of the peak responses of calcitriol and pre-calcitriol from the Standard solution
CS = concentration of USP Calcitriol RS in the Standard solution (mg/mL)
CU = concentration of Calcitriol in the Sample solution (mg/mL)
Acceptance criteria
Anhydrous form: 97.0%–103.0% on the solvent-free basis
Monohydrate form: 97.0%–103.0% on the anhydrous basis
4 IMPURITIES
Organic Impurities
Carry out the procedure as rapidly as possible, and protect all solutions containing calcitriol from light.
Buffer, Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of any individual impurity in the portion of Calcitriol taken:
Result = (rU/rT) × 100
rU = peak response of any individual peak other than the main calcitriol peak and the pre-calcitriol peak from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 1. The reporting threshold is 0.05%.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Triazoline adduct of pre-calcitriola | 0.43 | 0.1 |
| trans-Calcitriolb | 0.96 | 0.25 |
| Calcitriol | 1.0 | - |
| 1β-Calcitriolc | 1.15 | 0.1 |
| Methylene calcitriold | 1.5 | 0.25 |
| Any unspecified impurity | - | 0.1 |
| Total impurities | - | 1.0 |
a(6aR,7R,9aR)-11-[(3S,5R)-3,5-Dihydroxy-2-methylcyclohex-1-enyl]-7-[(R)-6-hydroxy-6-methylheptan-2-yl]-6a-methyl-2-phenyl 4a,5,6,6a,7,8,9,9a-octahydrocyclopenta[f][1,2,4]triazolo[1,2-a]cinnoline-1,3(2H,11H)-dione.
b(5E,7E)-9,10-Secocholesta-5,7,10(19)-triene-1α,3β,25-triol.
c(5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1β,3β,25-triol.
d(5Z,7E)-1α,3β-Dihydroxy-17-[(R)-7-hydroxy-7-methyloctan-2-yl]-9,10-secoandrosta-5,7,10(19)-triene.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ic: 3.5%–5.5%, where it is labeled as a monohydrate
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store as per labeling instructions.
Labeling: Where it is a monohydrate form, the label indicates.
USP Reference Standards 〈11〉
USP Calcitriol RS


