Calcipotriene
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
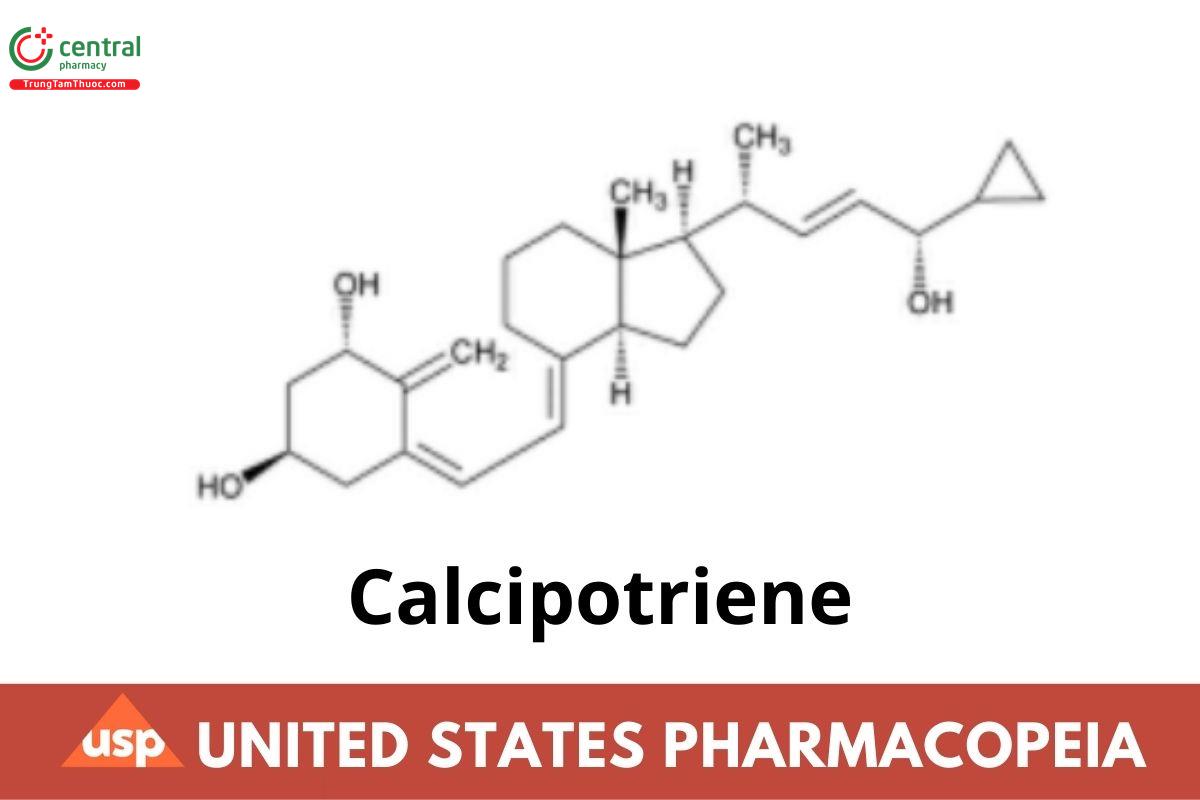
C27H40O3 412.60
9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1α,3β,5Z,7E,22E,24S)-;
(5Z,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol CAS RN®: 112965-21-6; UNII: 143NQ3779B. Monohydrate
C27H40O3.H2O 430.63 CAS RN®: 147657-22-5.
1 DEFINITION
Change to read:
Calcipotriene is anhydrous or contains one molecule of water of hydration. The anhydrous form contains NLT 97.0% and NMT 102.0% of calcipotriene (C27H40O3), calculated on the dried basis. The monohydrate form contains NLT 96.0% and NMT 102.0% of calcipotriene
(C27H40O3), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
Procedure
Protect solutions containing calcipotriene from light and air. Prepare the Standard solution and the Sample solution NMT 1 h before use. Prepare the System suitability solution daily.
Buffer: 1.0 g/L of tris(hydroxymethyl)aminomethane adjusted with phosphoric acid to a pH of 7.25 ± 0.25 Mobile phase: Acetonitrile and Buffer (45:55)
System suitability solution: 0.1 mg/mL of USP Calcipotriene RS and 0.01 mg/mL of USP Calcipotriene Related Compound C RS in Mobile phase
Standard solution: 0.1 mg/mL of USP Calcipotriene RS dissolved in 10% of acetonitrile and then diluted in Mobile phase Sample solution: 0.1 mg/mL of Calcipotriene dissolved in 10% of acetonitrile and then diluted in Mobile phase Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 264 nm
Column: 4.0-mm × 25-cm; 5-µm packing L7
Autosampler temperature: 4°
Flow rate: 1.0 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[Note-The relative retention times for calcipotriene related compound C and calcipotriene are 0.94 and 1.0, respectively.] Suitability requirements
Resolution: NLT 1.5 between calcipotriene related compound C and calcipotriene, System suitability solution Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of calcipotriene (C27H40O3) in the portion of Calcipotriene taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of calcipotriene from the Sample solution
rS = peak response of calcipotriene from the Standard solution
CS = concentration of USP Calcipotriene RS in the Standard solution (mg/mL)
CU = nominal concentration of calcipotriene in the Sample solution (mg/mL)
Acceptance criteria
Labeled as anhydrous form: 97.0%-102.0% on the dried basis
Labeled as monohydrate form: 96.0%-102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Change to read:
Organic Impurities, Procedure 1: HPLC
Protect solutions containing calcipotriene from light and air. Prepare the Standard solution and the Sample solution NMT 1 h before use. Prepare the System suitability solution daily.
Buffer, Mobile phase, System suitability solution, and Chromatographic system : Proceed as directed in the Assay. Standard stock solution: Use the Standard solution in the Assay.
Standard solution: 0.004 mg/mL of USP Calcipotriene RS in Mobile phase, from the Standard stock solution
Sensitivity solution: 0.2 µg/mL of USP Calcipotriene RS in Mobile phase, from Standard solution Sample solution: 0.4 mg/mL of Calcipotriene dissolved in 10% of acetonitrile and then diluted in Mobile phase
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note-The relative retention times for calcipotriene related compound C and calcipotriene are 0.94 and 1.0, respectively.] Suitability requirements
Resolution: NLT 1.5 between calcipotriene related compound C and calcipotriene, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Result = (rU/rS) x (CS/CU) × 100
rU = ppeak response of any impurity from the Sample solution
rS = peak response of calcipotriene from the Standard solution
CS = concentration of USP Calcipotriene RS in the Standard solution (mg/mL)
CU = concentration of Calcipotriene in the Sample solution (mg/mL)
Calculate the percentage of any impurity in the portion of Calcipotriene taken:
Acceptance criteria: See Table 1. The reporting threshold is 0.05%.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Calcipotriene impurity Ba | 0.83 | 0.5 |
| Calcipotriene related compound Cb | 0.92–0.96 | 1.0 |
| Calcipotriene | 1.00 | - |
| Calcipotriene impurity Dc | ||
| Any individual unspecifi ed impurity | - | 0.10 |
| Total impurities | - | 2.5 |
a(5Z,7Z,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol.
b(5E,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol.
c(5Z,7E,22E,24R)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol.
Change to read:
Organic Impurities, Procedure 2: TLC
Prepare solutions containing calcipotriene in low-actinic glassware, and protect from air. Carry out the test as rapidly as possible. Diluent: Chloroform and triethylamine (9:1)
System suitability solution: 10 mg/mL of USP Calcipotriene RS in Diluent. Heat in a water bath at 60° for 2 h to form pre-Calcipotriene.
Standard solution 1: 0.025 mg/mL of USP Calcipotriene RS in Diluent (0.25%)
Standard solution 2: 0.01 mg/mL of USP Calcipotriene RS in Diluent (0.10%)
Standard solution 3: 0.05 mg/mL of USP Calcipotriene RS in Diluent (0.5%)
Sample solution: 10 mg/mL of Calcipotriene in Diluent
Developing solvent system: Methylene chloride and isobutyl alcohol (80:20)
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic plate coated with silica gel mixture
Application volume: 10 µL
Spray reagent: Transfer 20 mL of sulfuric acid into a 100-mL volumetric ask, and dilute with alcohol to volume.
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: The secondary spot pre-Calcipotriene and principle spot calcipotriene are clearly separated. Analysis
Samples: Standard solution 1, Standard solution 2, Standard solution 3, and Sample solution
Develop with Developing solvent system until the solvent system has moved two-thirds of the plate from the point of spotting. Remove the plate, and let the plate air-dry. Dry it again at 140° for 10 min followed by spraying the hot plate with the Spray reagent. Dry the plate for NMT 1 min at 140°. Examine the plate under UV light at 366 nm.
Acceptance criteria: The spot of any impurity in the Sample solution is not more intense than the spot of calcipotriene in the appropriate Standard solution specified in Table 2.
Table 2
| Name | Relative Retardation (Rret) | Comparison Solution | Acceptance Criteria, NMT (%) |
| Calcipotriene impurity Ga and calcipotriene impurity Hb | 0.4 | Standard solution 1 | 0.25 |
| pre-Calcipotriene c | 0.9 | Standard solution 3 | 0.5 |
| Calcipotriene | 1.0 | - | - |
| Calcipotriene impurity Ad | 1.2 | Standard solution 1 | 0.25 |
| Any other individual impurity | - | Standard solution 2 | 0.10 |
a 24,24′-Oxybis[(5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β-diol].
b (5Z,7E,22E,24R)-24-Cyclopropyl-24-{[(5Z,7E,22E,24S)-24-cyclopropyl-1α,3β-dihydroxy-9,10-secochola-5,7,10(19),22-tetraen-24-yl]oxy}-9,10- secochola-5,7,10(19),22-tetraene-1α,3β-diol.
c (5E,6E,22E,24S)-24-Cyclopropyl-9,10-secochola-5(10),6,22-triene-1α,3β,24-triol.
d (5Z,7E ,22E)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-24-one-1α,3β-diol.
5 SPECIFIC TESTS
Change to read:
Loss on Drying (where it is labeled as anhydrous form)
(See Thermal Analysis 〈891〉.)
Sample: 5 mg
Analysis: Heat the Sample to 105° at a rate of 10°/min, and hold at 105° for 60 min.
Acceptance criteria: NMT 1.0%
Add the following:
Water Determination 〈921〉, Method I(where it is labeled as monohydrate form): 3.3%-5.0%
6 ADDITIONAL REQUIREMENTS
Change to read:
Packaging and Storage: Preserve in tight containers. If labeled as anhydrous form, store at 2°-8° or at −20° or below. If labeled as monohydrate, store at room temperature. Protect from light.
Add the following:
Labeling: Label it to indicate whether it is the anhydrous form or the monohydrate form.
Change to read:
USP Reference Standards 〈11〉
USP Calcipotriene RS
USP Calcipotriene Related Compound C RS
(5E,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol.
C27H40O3 412.61


