Calcifediol
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
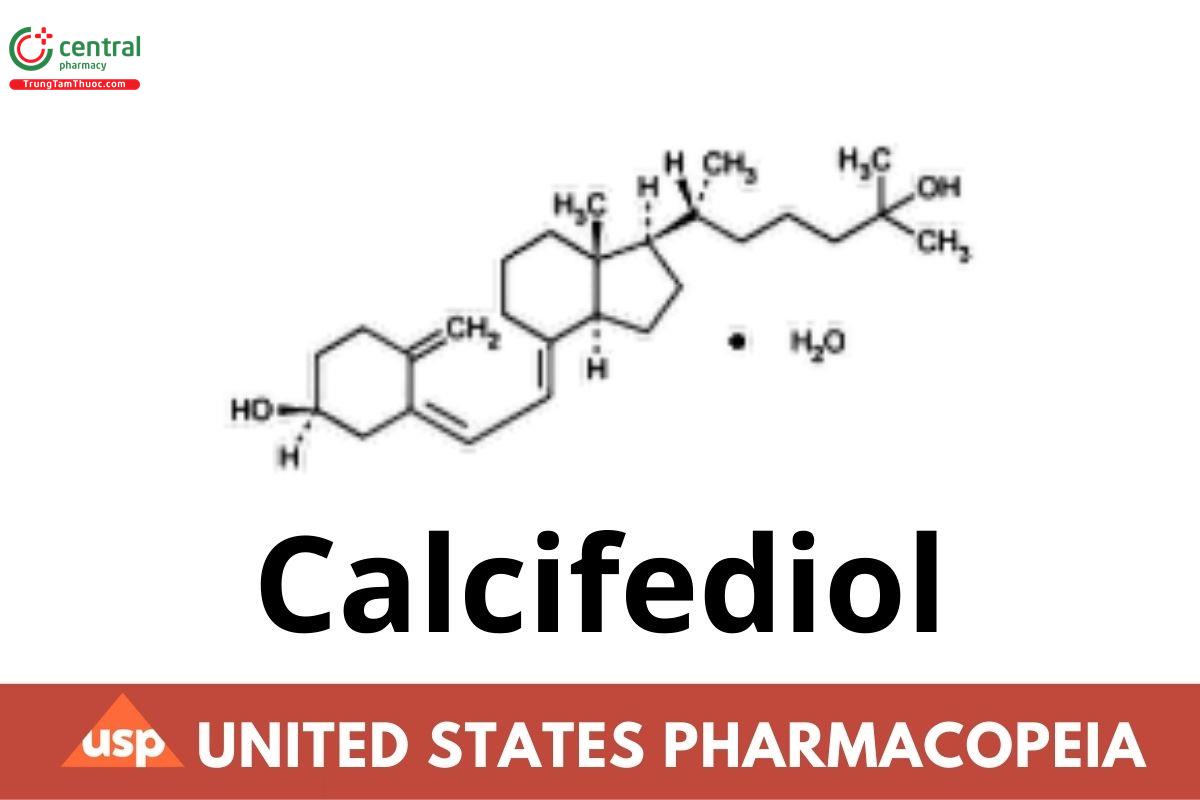
C27H44O2.H2O. 418.65
9,10-Secocholesta-5,7,10(19)-triene-3,25-diol monohydrate, (3β,5Z,7E).
25-Hydroxycholecalciferol monohydrate CAS RN®: 63283-36-3; UNII: P6YZ13C99Q.
Calcifediol contains not less than 97.0 percent and not more than 103.0 percent of C27H44O2.H2O
Packaging and storage - Preserve in tight, light-resistant containers at controlled room temperature.
USP Reference standards 〈11〉
USP Calcifediol RS
Change to read:
Identification, Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M (CN 1-May-2020)
Water Determination, Method Ia 〈921〉: between 3.8% and 5.0%, determined on a 0.2-g specimen.
Assay
Internal standard solution-Dissolve Testosterone in ethyl acetate to obtain a solution having a concentration of about 0.10 mg per mL. Mobile phase-Prepare a suitable degassed solution of about 6 volumes of heptane, 6 volumes of water-saturated heptane, 3 volumes of methylene chloride, and 5 volumes of ethyl acetate.
Standard preparation-Dissolve an accurately weighed quantity of USP Calcifediol RS in Internal standard solution, and dilute quantitatively and stepwise with Internal standard solution to obtain a solution having a known concentration of about 20 µg per mL.
Assay preparation-Transfer about 10 mg of Calcifediol, accurately weighed, to a 50-mL volumetric flask, dissolve in Internal standard solution, dilute with Internal standard solution to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with Internal standard solution to volume, and mix.
Chromatographic system (see Chromatography 〈621〉)-The liquid chromatograph is equipped with a 4-mm × 30-cm column that contains packing L3, a detector that monitors absorption at 254 nm, and a pump capable of providing constant ow up to a minimum of 2000 psi. System suitability-The relative standard deviation for peak response ratios for four replicate injections of Standard preparation is not more than 3.0%, and the resolution factor is not less than 3.0.
Procedure-Introduce equal volumes of the Standard preparation and the Assay preparation into the chromatograph (see Chromatography〈621), and measure the peak responses obtained. Calculate the quantity, in mg, of C27H44O2.H2O. in the portion of Calcifediol taken by the formula:
0.5C(RU/RS)
in which C is the concentration, in µg per mL, of USP Calcifediol RS in the Standard preparation; and RU and RS are the ratios of the peak responses of calcifediol to testosterone obtained from the Assay preparation and the Standard preparation, respectively.


