Butylated Hydroxyanisole
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
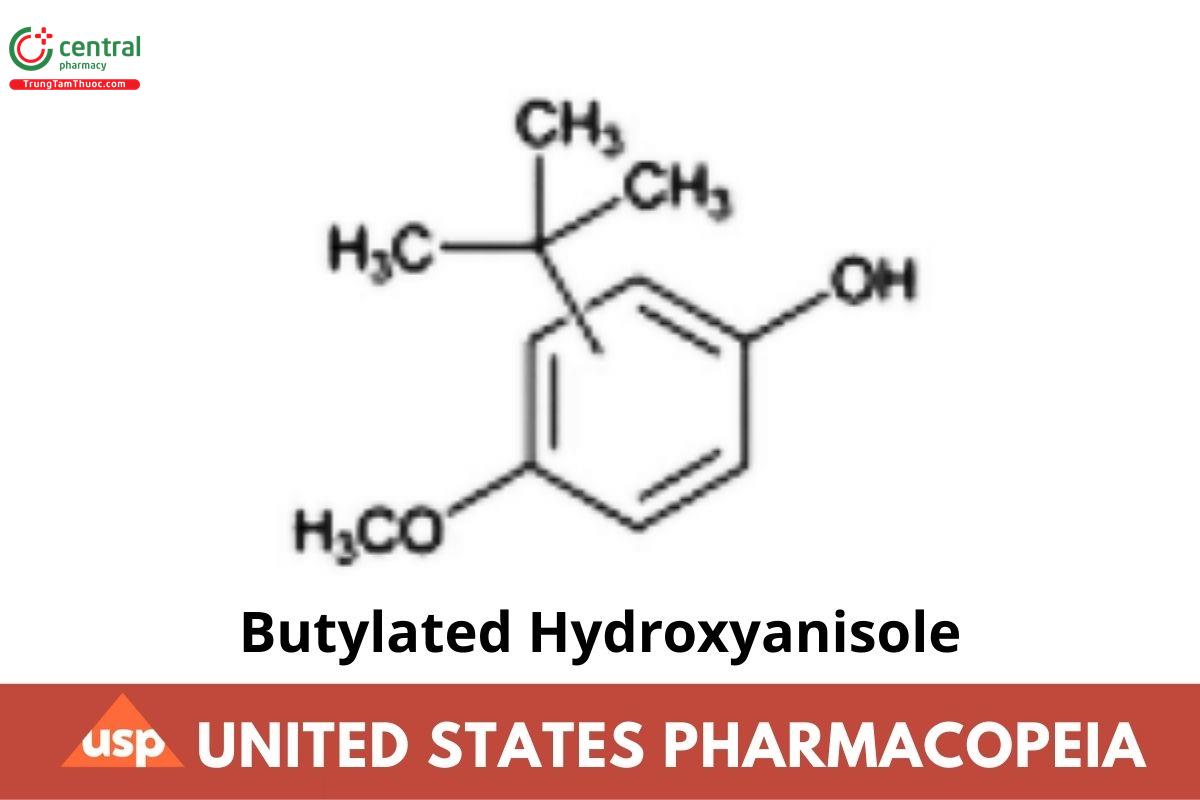
C11H16O2 180.24
Phenol, (1,1-dimethylethyl)-4-methoxy-;
tert-Butyl-4-methoxyphenol CAS RN®: 25013-16-5.
1 DEFINITION
Butylated Hydroxyanisole is predominantly 3-tert-butyl-4-hydroxyanisole, with varying amounts of 2-tert-butyl-4-hydroxyanisole. It contains NLT 98.5% of butylated hydroxyanisole (C11H16O2) as a sum of the two isomers.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A (CN 1-May-2020)
Analysis: Determine the position of the most intense 3-tert-butyl-4-hydroxyanisole peaks within 10 cm–1 of 682, 815, 855, 914, 1031, 1196, 1413, and 1504 cm–1 in a spectrum of USP 3-tert-Butyl-4-hydroxyanisole RS. Compare the peak positions of Butylated Hydroxyanisole to those of USP 3-tert-Butyl-4-hydroxyanisole RS.
Acceptance criteria: All peak positions determined from Butylated Hydroxyanisole are within 5 cm–1 of those determined from USP 3-tert Butyl-4-hydroxyanisole RS.
B.
Solution A: 5% acetic acid, prepared by diluting 50 mL of glacial acetic acid in a 1-L ask with water to volume Mobile phase: Acetonitrile and Solution A (65:35)
Standard solution: 0.4 mg/mL of USP 3-tert-Butyl-4-hydroxyanisole RS and 0.1 mg/mL of USP 2-tert-Butyl-4-hydroxyanisole RS in Mobile phase
Sample solution: 0.5 mg/mL of Butylated Hydroxyanisole in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 275 nm
Column: 3.0-mm × 15-cm; 3-µm packing L1
Column temperature: 40°
Flow rate: 0.75 mL/min
Injection volume: 10 µL
Run time: NLT 15 min
Analysis
Samples: Standard solution and Sample solution
[Note—2-tert-Butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole coelute under these chromatographic conditions. However, a small shoulder of 2-tert-butyl-4-hydroxyanisole may be seen on the left-hand side of the 3-tert-butyl-4-hydroxyanisole peak. The retention time of the 3-tert-butyl-4-hydroxyanisole peak is about 2.1 min.]
Acceptance criteria: The retention time of the main peak of the Sample solution corresponds to that of the Standard solution. The chromatographic prole of the Sample solution should be similar to that of the Standard solution and exhibit only 1 major peak corresponding to butylated hydroxyanisole.
3 ASSAY
Change to read:
Procedure
Solution A: Prepare as directed in Identication B.
Mobile phase: Acetonitrile and Solution A (45:55)
System suitability solution: (NF 1-May-2019) 90 µg/mL of USP 3-tert-Butyl-4-hydroxyanisole RS and 10 µg/mL of USP 2-tert-Butyl-4- hydroxyanisole RS in Mobile phase
Standard solution A: 90 µg/mL of USP 3-tert-Butyl-4-hydroxyanisole RS in Mobile phase
Standard solution B: 10 µg/mL of USP 2-tert-Butyl-4-hydroxyanisole RS in Mobile phase (NF 1-May-2019)
Sample solution: 100 µg/mL of Butylated Hydroxyanisole in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 290 nm
Column: 4.6-mm × 75-mm; 3.5-µm packing L1
Column temperature: 30°
Flow rate: 1.2 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution, Standard solution A, and Standard solution B (NF 1-May-2019)
[Note—The retention times of 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole are about 4.2 and 4.6 min, respectively.] Suitability requirements
Resolution: NLT 1.5 between the 3-tert-butyl-4-hydroxyanisole isomer and 2-tert-butyl-4-hydroxyanisole isomer peaks, System suitability solution (NF 1-May-2019)
Tailing factor: NMT 1.5, Standard solution A and Standard solution B (NF 1-May-2019)
Relative standard deviation: NMT 2.0% for the 3-tert-butyl-4-hydroxyanisole isomer and 2-tert-butyl-4-hydroxyanisole isomer peaks,
Standard solution A and Standard solution B (NF 1-May-2019)
Analysis
Samples: Standard solution A, Standard solution B, (NF 1-May-2019) and Sample solution
Measure the peak areas for each isomer.
Calculate the percentage of each isomer in the portion of Butylated Hydroxyanisole taken:
Result = (rU/rS) × (CS/CU) × 100
= peak area of the corresponding isomer from the Sample solution
= peak area of the corresponding isomer from Standard solution A or Standard solution B (NF 1-May-2019)
= concentration of the appropriate Reference Standard in Standard solution A or Standard solution B (NF 1-May-2019) (µg/mL)
= concentration of Butylated Hydroxyanisole in the Sample solution (µg/mL)
[Note—Calculate the percentage of butylated hydroxyanisole (C11H16O2) in the portion of Butylated Hydroxyanisole taken by adding the quantities of the two isomers.]
Acceptance criteria: NLT 98.5%
4 IMPURITIES
Residue on Ignition 〈281〉
Sample: 10 g
Acceptance criteria: NMT 0.01%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP 2-tert-Butyl-4-hydroxyanisole RS C11H16O2 180.25
USP 3-tert-Butyl-4-hydroxyanisole RS C11H16O2 180.25


