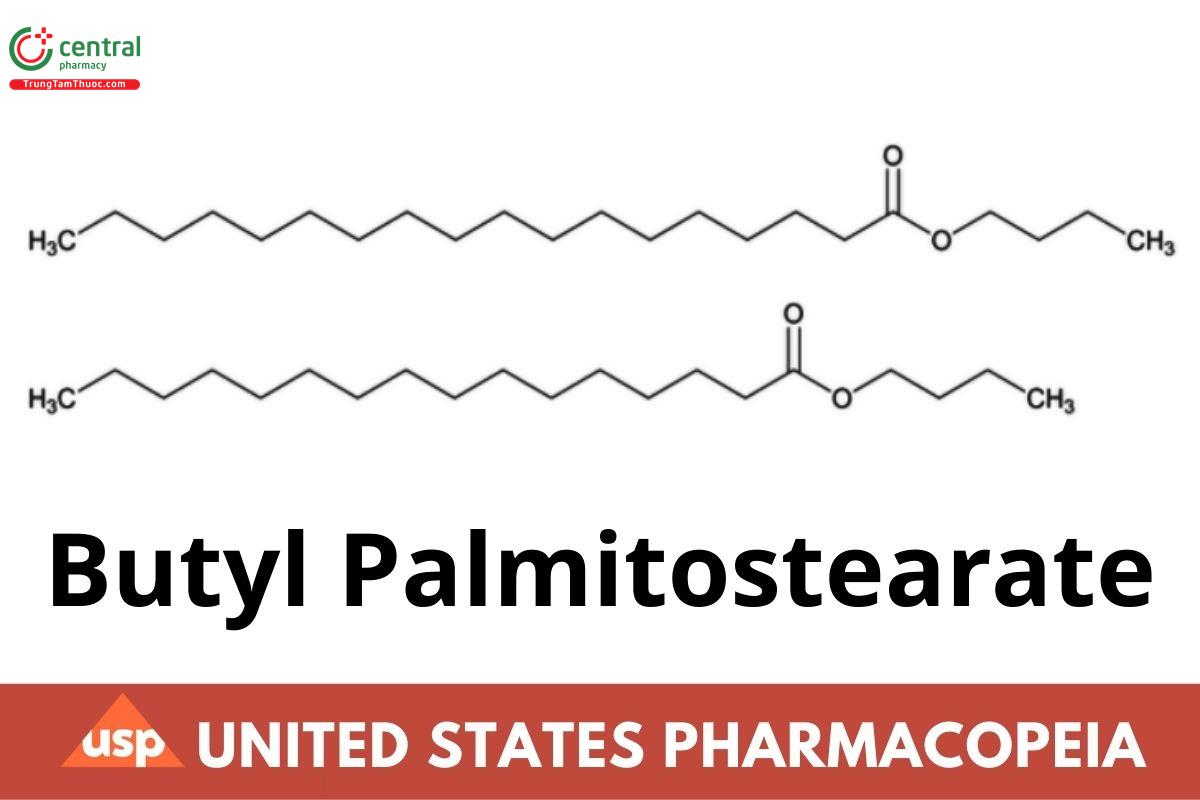
Butyl Palmitostearate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Butyl Palmitostearate is a mixture of the butyl ester of stearic acid (C16H32O2) and the butyl ester of palmitic acid (C18H36O2). It contains 40.0%– 80.0% of butyl stearate (C22H44O2). The total percentage of butyl stearate (C22H44O2) and butyl palmitate (C20H40O2) is NLT 90.0%.
2 IDENTIFICATION
A. It meets the requirements of the test for Content of Butyl Palmitate and Butyl Stearate.
3 ASSAY
Content of Butyl Palmitate and Butyl Stearate
Standard solution A: 2.0 mg/mL of USP Butyl Stearate RS in chloroform
Standard solution B: 1.0 mg/mL of USP Butyl Palmitate RS in chloroform
System suitability solution: 1.4 µg/mL of USP Butyl Stearate RS and 1.0 µg/mL of USP Butyl Palmitate RS in chloroform, prepared from Standard solution A, Standard solution B, and chloroform
Sample solution: 2.0 mg/mL of Butyl Palmitostearate in chloroform
4 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m capillary; bonded with a 0.25-µm layer of thickness of phase G27
Temperatures
Detector: 280°
Injection port: 280°
Column: See Table 1.
Table 1
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
60 | — | 60 | 2 |
60 | 4 | 250 | 20.5 |
Carrier gas: Helium
Flow rate: 1.4 mL/min
Injection volume: 1 µL
Injection type: Split injection, split ratio is 5:1
4.1 System suitability
Samples: Standard solution A and System suitability solution
[Note—The relative retention times for butyl palmitate and butyl stearate are 0.91 and 1.00, respectively.]
System suitability requirements
Resolution: NLT 20 between butyl palmitate and butyl stearate, System suitability solution
Tailing factor: NMT 2.0 for the butyl stearate peak, System suitability solution
Relative standard deviation: NMT 3.0% for the butyl stearate peak, Standard solution A
4.2 Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Identify the butyl palmitate and butyl stearate peaks in the Sample solution based on those in Standard solution A and Standard solution B. Calculate the percentage of butyl palmitate or butyl stearate in the portion of Butyl Palmitostearate taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of butyl palmitate or butyl stearate from the Sample solution
rS = peak response of butyl palmitate from Standard solution B or peak response of butyl stearate from Standard solution A
CS = concentration of USP Butyl Palmitate RS in Standard solution B or concentration of USP Butyl Stearate RS in Standard solution A
CU = concentration of Butyl Palmitostearate in the Sample solution
4.3 Acceptance criteria
Content of butyl stearate: 40.0%–80.0%
Sum of butyl stearate and butyl palmitate: NLT 90.0%
5 SPECIFIC TESTS
Melting Range or Temperature, Class II〈741〉: 17°–24°
Fats and Fixed Oils, Acid Value〈401〉: NMT 0.5
Fats and Fixed Oils, Iodine Value〈401〉: NMT 1.0
Fats and Fixed Oils, Saponification Value〈401〉: 165–180
Water Determination, Method Ia〈921〉: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature. Keep away from heat and sources of ignition.
USP Reference Standards 〈11〉
USP Butyl Palmitate RS
USP Butyl Stearate RS


