Butabarbital Sodium
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
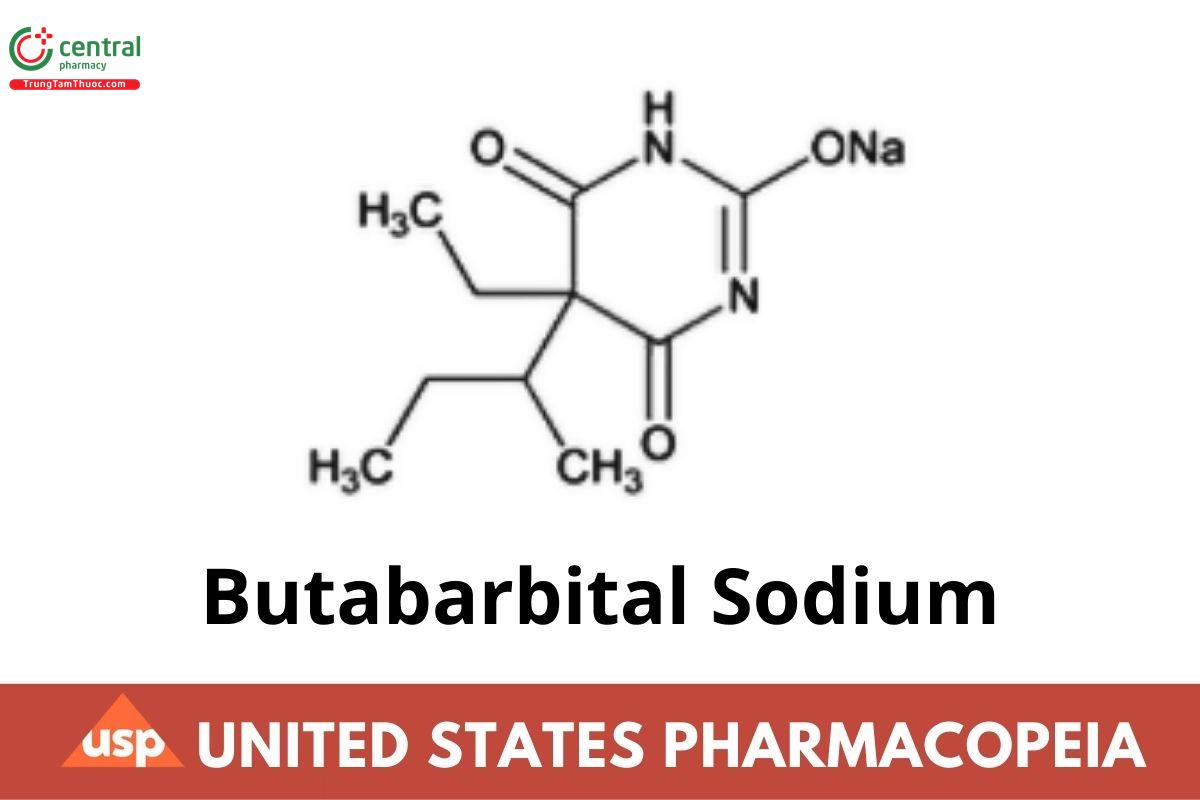
C10H15N2NaO3 234.23
2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)-, monosodium salt; Sodium 5-sec-butyl-5-ethylbarbiturate CAS RN®: 143-81-7; UNII: 9WTD501918.
1 DEFINITION
Butabarbital Sodium contains NLT 98.2% and NMT 100.5% of butabarbital sodium (C10H15N2NaO3), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
2.1 A. ASPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
Sample: 150 mg of Butabarbital Sodium
Analysis: Transfer the Sample to a suitable separator, dissolve in 10 mL of water, and add 15 mL of 3 N hydrochloric acid. Extract with three 20-mL portions of chloroform, filter the extracts through anhydrous sodium sulfate, and collect the extracts in a suitable beaker. Evaporate the combined extracts on a steam bath with the aid of a current of air to dryness, and dry the residue at 105° for 2 h.
Acceptance criteria: Meets the requirements
Change to read:
2.2 B. ASPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970 (CN 1-MAY-2020)
Analytical wavelength: 240 nm
Buffer: pH 9.6 alkaline borate buffer (see Reagents, Indicators, and Solutions-Buffer Solutions)
Standard solution: 10 µg/mL of USP Butabarbital RS in Buffer
Sample solution: 10 µg/mL of Butabarbital Sodium in Buffer
Acceptance criteria: Absorptivities, calculated on the dried basis, do not differ by more than 3.0%.
2.3 C. IDENTIFICATION TESTS GENERAL, Sodium (191)
Sample: 100 mg of Butabarbital Sodium
Analysis: Ignite the Sample, and proceed as directed in the chapter using the residue.
Acceptance criteria: Meets the requirements
3 ASSAY
3.1 PROCEDURE
Buffer: pH 9.6 alkaline borate buffer (see Reagents, Indicators, and Solutions-Buffer Solutions)
Standard stock solution: 0.125 mg/mL of USP Butabarbital RS in Buffer
Standard solution: 0.0125 mg/mL of USP Butabarbital RS from Standard stock solution in Buffer
Sample stock solution: 0.140 mg/mL of Butabarbital Sodium in Buffer
Sample solution: 0.0140 mg/mL from Sample stock solution in Buffer
Instrumental conditions
Mode: UV
Analytical wavelength: Maximum absorbance at about 240 nm
Blank: Buffer
Analysis
Samples: Standard solution, Sample solution, and Blank
Concomitantly determine the absorbances of the solutions.
Calculate the percentage of butabarbital sodium (C10H15N2NaO3) in the portion of Butabarbital Sodium taken:
Result = (AU/AS) × (CS/CU) × (Mr1/Mr2) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of USP Butabarbital RS in the Standard solution (µg/mL)
CU = concentration of Butabarbital Sodium in the Sample solution (µg/mL)
Mr1 = molecular weight of butabarbital sodium, 234.23
Mr2 = molecular weight of butabarbital, 212.25
Acceptance criteria: 98.2%-100.5% on the dried basis
4 IMPURITIES
4.1 ORGANIC IMPURITIES
Diluent: Chloroform and methanol (50:50)
Standard solution A: 4.0 mg/mL of USP Butabarbital RS in Diluent
Standard solution B: 0.4 mg/mL of USP Butabarbital RS from Standard solution A in Diluent
Sample solution: 44 mg/mL of Butabarbital Sodium in Diluent
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Acetone, methylene chloride, methanol, and ammonium hydroxide (5:3:1:1)
Spray reagent: A solution of mercurous nitrate dihydrate in 0.15 N nitric acid (1 in 100)
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Develop the chromatogram in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, and dry under a current of air. Spray the plate with Spray reagent, and immediately estimate the intensities of any spots of the Sample solution, other than the principal spot, in comparison with Standard solution B.
Acceptance criteria: The Re value of the principal spot of the Sample solution corresponds to that of Standard solution A; and the sum of the intensities of any secondary spots of the Sample solution is NMT the intensity of the principal spot produced by Standard solution B, corresponding to NMT a total of 1% of impurities.
5 SPECIFIC TESTS
5.1 COMPLETENESS OF SOLUTION
Sample solution: Dissolve 1.0 g of Butabarbital Sodium in 10 mL of carbon dioxide-free water.
Acceptance criteria: After 1 min, the solution is clear and free from undissolved solid.
5.2 PH (791)
Sample solution: Use the Sample solution from the test for Completeness of Solution.
Acceptance criteria: 10.0-11.2
5.3 LOSS ON DRYING (731)
Analysis: Dry at 150° to constant weight.
Acceptance criteria: NMT 5.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11).
USP Butabarbital RS


