Bromocriptine Mesylate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
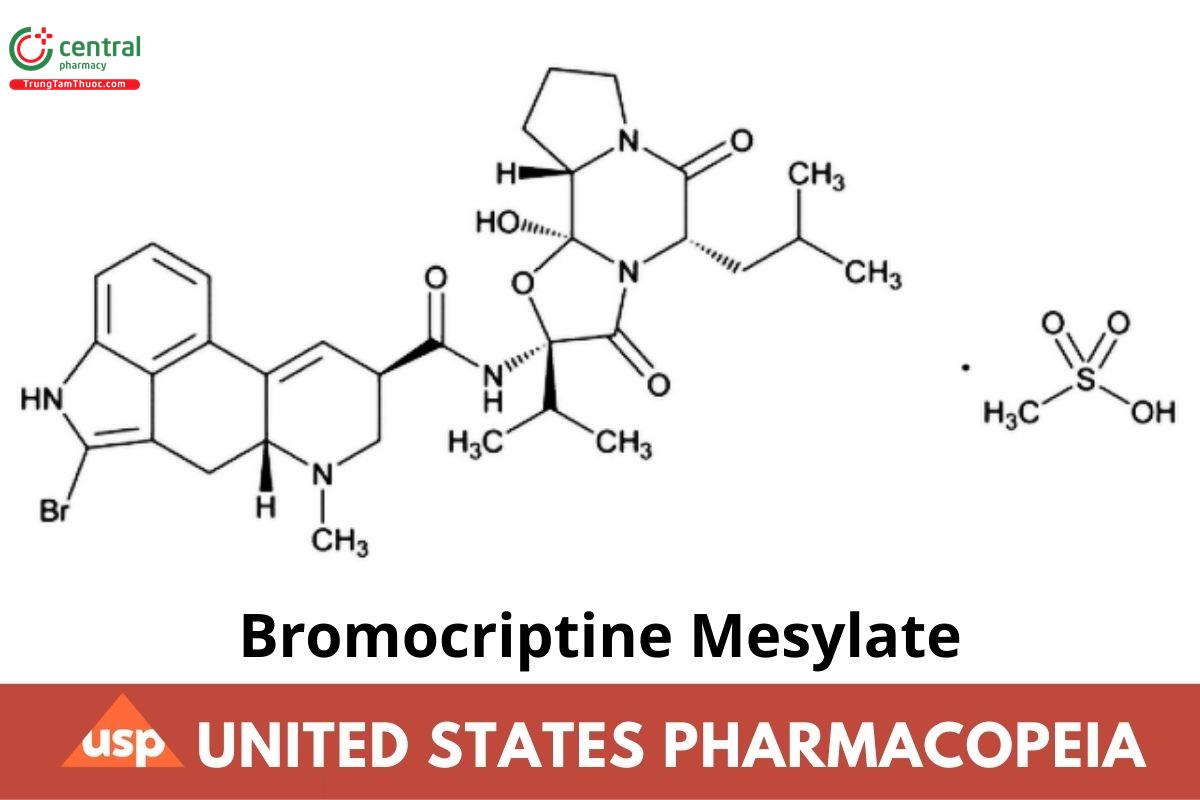
C32H40BrN5O5 · CH4SO3 750.70
Ergotaman-3',6',18-trione, 2-bromo-12-hydroxy-2'-(1-methylethyl)-5-(2-methylpropyl)-, monomethanesulfonate (salt), (5')-;
2-Bromoergocryptine monomethanesulfonate (salt) CAS RN: 22260-51-1; UNII: FFP983J3OD.
1 DEFINITION
Bromocriptine Mesylate contains NLT 98.0% and NMT 102.0% of C32H40BrN5O5 · CH4SO3 calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M (CN 1-Mar-2020): Undried
Change to read:
B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970 (CN 1-May-2020)
Sample solution: 50 µg/mL in 0.1 M methanolic methanesulfonic acid
Acceptance criteria: Meets the requirements
3 ASSAY
PROCEDURE
Sample solution: 600 mg of Bromocriptine Mesylate
Analysis: Dissolve with 80 mL of a mixture of acetic anhydride and glacial acetic acid (7:1). Titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction (see Titrimetry (541)). Each mL of 0.1 N perchloric acid is equivalent to 75.07 mg of C32H40BrN5O5
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
INORGANIC IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
ORGANIC IMPURITIES
4.1 PROCEDURE 1: LIMIT OF METHANESULFONIC ACID CONTENT
Sample solution: 400 mg of Bromocriptine Mesylate
Analysis: Dissolve with 70 mL of methanol. Titrate under nitrogen with 0.1 N methanolic potassium hydroxide VS. Perform a blank determination, and make any necessary correction (see Titrimetry (541)). Each mL of 0.1 N methanolic potassium hydroxide is equivalent to 9.61 mg of CH3SO3H.
Acceptance criteria: NLT 12.5% and NMT 13.4% of CH3SO3H on the dried basis
4.2 PROCEDURE 2
Solution A: 0.1 N citric acid solution. Adjust with hydrochloric acid to a pH of 2.0.
Diluent: Methanol and Solution A (1:1)
Solution B: Acetonitrile and 0.01 M phosphate buffer, pH 7.0 (2:3)
Solution C: Acetonitrile and 0.01 M phosphate buffer, pH 7.0 (3:2)
Mobile phase: See the gradient table below.
Time (min) | Solution B (%) | Solution C (%) |
| 0 | 100 | 0 |
| 18 | 100 | 0 |
| 30 | 0 | 100 |
| 40 | 0 | 100 |
| 41 | 100 | 0 |
System suitability solution: 2.0 mg/mL each of a-ergocryptine and Bromocriptine Mesylate in Diluent
Standard stock solution: 46 µg/mL of USP Bromocriptine Mesylate RS in methanol and Solution A (1:1). [NOTE-Dissolve in 50% of the flask volume of methanol and dilute with Solution A to volume.]
Standard solution: 4.6 µg/mL of USP Bromocriptine Mesylate RS in Diluent from the Standard stock solution
Sample solution: 4.6 mg/mL of Bromocriptine Mesylate in methanol and Solution A (1:1). [NOTE-Dissolve in 50% of the flask volume of methanol and dilute with Solution A to volume.]
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 300 nm
Column: 4.6-mm x 15-cm; 3-um packing L1
Flow rate: 2 mL/min
Injection size: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for a-ergocryptine and bromocriptine mesylate are 0.46 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 15 between a-ergocryptine and bromocriptine mesylate, System suitability solution
Tailing factor: NMT 1.5, System suitability solution
Relative standard deviation: NMT 10.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
[NOTE-The relative retention times for bromocriptine and bromocriptinine are 1.0 and 1.7, respectively.]
Calculate the percentage of each impurity in the portion of Bromocriptine Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of bromocriptine from the Standard solution
Cs = concentration of USP Bromocriptine Mesylate RS in the Standard solution (mg/mL)
Cu = concentration of Bromocriptine Mesylate in the Sample solution (mg/mL)
F = relative response factor equal to 1.4 for any peak eluting at a relative retention time of about 0.9 or less, and equal to 1.0 for all other peaks
Acceptance criteria
Individual impurities: NMT 0.4% of bromocriptinine is found; NMT 0.1% of any individual impurity is found.
Total impurities: NMT 1.0%
5 SPECIFIC TESTS
Color of Solution 〈631〉
Matching solutions: Prepare three solutions, A, B, and C, containing, respectively, the following parts of cobaltous chloride CS, ferric chloride
CS, cupric sulfate CS, and dilute hydrochloric acid (1 in 40).
A: 3.0:3.0:2.4:31.6
B: 1.0:2.4:0.4:36.2
C: 0.6:2.4:0:37.0
Sample solution: 10 mg/mL of Bromocriptine Mesylate in methanol
Analysis: Compare the Sample solution with 10-mL portions of the Matching solutions in suitable matched tubes.
Acceptance criteria: The solution is clear and not darker in color than Matching solutions A, B, and C.
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL, in a mixture of methylene chloride and methanol (1:1)
Acceptance criteria: +95° to +105°
Loss on Drying
(See Thermal Analysis 〈891〉.)
Analysis: Determine the percentage of volatile substances by thermogravimetric analysis using 10 mg of Bromocriptine Mesylate. Heat the specimen under test at the rate of 10°/min in an atmosphere of nitrogen at a ow rate of 45 mL/min. Record the thermogram from ambient temperature to 160°.
Acceptance criteria: It loses NMT 4.0% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers, in a cold place.
USP Reference Standards 〈11〉
USP Bromocriptine Mesylate RS


