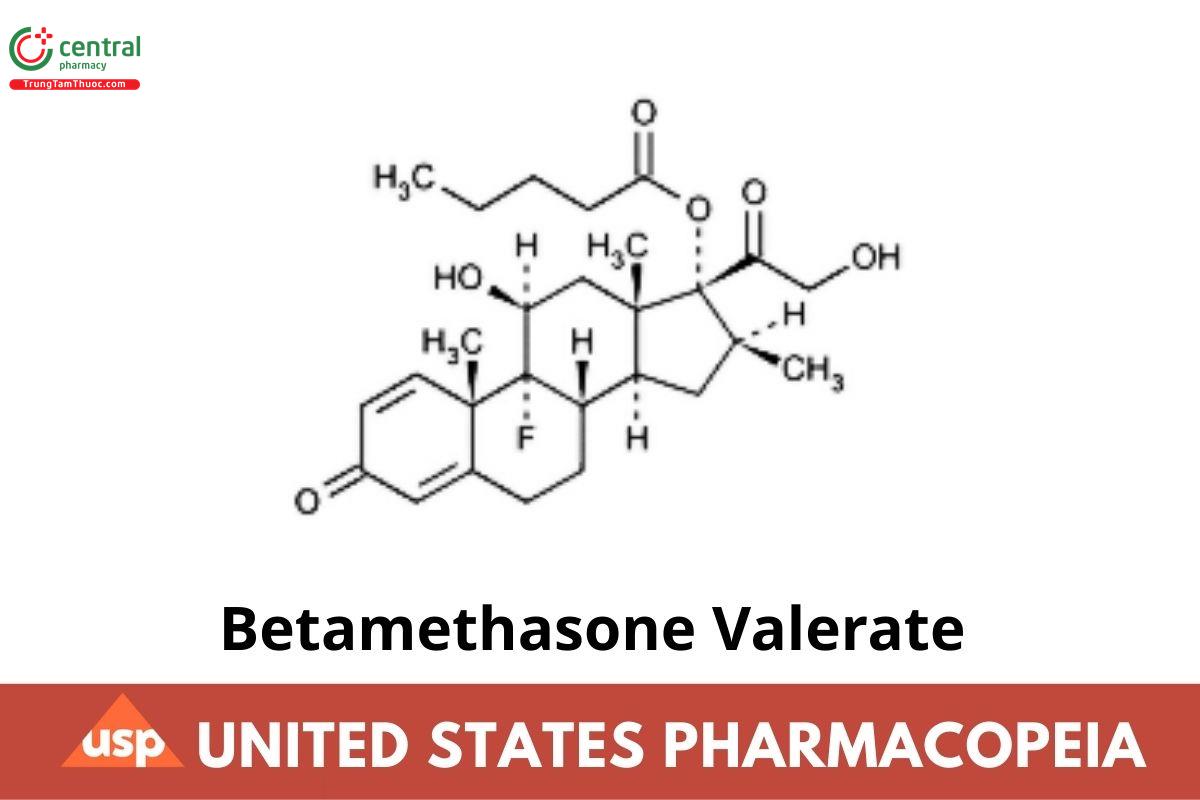
Betamethasone Valerate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Betamethasone Valerate contains NLT 97.0% and NMT 103.0% of betamethasone valerate (C₂₇H₃₇FO₆), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M or 197A (USP 1-Aug-2024)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP 1-Aug-2024)
3 ASSAY
Procedure
Mobile phase: Acetonitrile and water (50:50). [Note—The mobile phase composition should be tightly controlled (±2%) to maintain the elution order and resolution between specified and unspecified impurities.]
System suitability solution: 250 μg/mL of USP Betamethasone Valerate System Suitability Mixture RS and 2.5 µg/mL of USP Betamethasone Valerate Related Compound D RS in Mobile phase. Sonicate to dissolve.
Standard solution: 250 μg/mL of USP Betamethasone Valerate RS in Mobile phase. Sonicate to dissolve.
Sample solution: 250 μg/mL of Betamethasone Valerate in Mobile phase. Sonicate to dissolve.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Temperatures
Autosampler: 4°
Column: 30°
Flow rate: 1.2 mL/min
Injection volume: 10 µL
Run time: NLT 2 times the retention time of betamethasone valerate
System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 1 for the relative retention times.]
Suitability requirements
Resolution: NLT 5.0 between betamethasone valerate and betamethasone valerate related compound H;
NLT 1.5 between betamethasone valerate related compound H and betamethasone valerate related compound D, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 1.10%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of betamethasone valerate (C₂₇H₃₇FO₆) in the portion of Betamethasone Valerate taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of betamethasone valerate from the Sample solution
rₛ = peak response of betamethasone valerate from the Standard solution
Cₛ = concentration of USP Betamethasone Valerate RS in the Standard solution (μg/mL)
Cᵤ = concentration of Betamethasone Valerate in the Sample solution (μg/mL)
(USP 1-Aug-2024)
Acceptance criteria: 97.0%–103.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉
Analysis: Use a platinum crucible.
Acceptance criteria: NMT 0.2%
Limit of Betamethasone Valerate Related Compound H
Mobile phase: Acetonitrile and water (40:60)
Diluent: Acetonitrile and water (50:50)
System suitability solution: 250 µg/mL of USP Betamethasone Valerate System Suitability Mixture RS in Diluent. Sonicate to dissolve.
Standard solution: 0.375 µg/mL of USP Betamethasone Valerate RS in Diluent
Sample solution: 250 µg/mL of Betamethasone Valerate in Diluent. Sonicate to dissolve.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Temperatures
Autosampler: 4°
Column: 30°
Flow rate: 1.4 mL/min
Injection volume: 75 µL
Run time: NLT 3 times the retention time of betamethasone valerate
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for betamethasone valerate and betamethasone valerate related compound H are 1.0 and 1.36, respectively.]
Suitability requirements
Resolution: NLT 7.5 between betamethasone valerate and betamethasone valerate related compound H, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of betamethasone valerate related compound H in the portion of Betamethasone Valerate taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × (1/F) × 100
rᵤ = peak response of betamethasone valerate related compound H from the Sample solution
rₛ = peak response of betamethasone valerate from the Standard solution
Cₛ = concentration of USP Betamethasone Valerate RS in the Standard solution (µg/mL)
Cᵤ = concentration of Betamethasone Valerate in the Sample solution (µg/mL)
F = relative response factor, 0.95
Acceptance criteria: NMT 0.15% (USP 1-Aug-2024)
Organic Impurities
[Note—Solutions containing betamethasone valerate should be prepared fresh and injected within 4 h.]
Mobile phase and System suitability solution: Prepare as directed in the Assay.
Sensitivity solution: 0.12 µg/mL of USP Betamethasone Valerate RS in Mobile phase
Standard solution: 1.75 µg/mL of USP Betamethasone RS, 0.25 µg/mL of USP Betamethasone Valerate RS, and 0.75 µg/mL of USP Betamethasone Valerate Related Compound A RS in Mobile phase
Sample solution: 250 µg/mL of Betamethasone Valerate in Mobile phase. Sonicate to dissolve.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Temperatures
Autosampler: 4°
Column: 30°
Flow rate: 1.2 mL/min
Injection volume: 35 µL
Run time: NLT 2 times the retention time of betamethasone valerate
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
Suitability requirements
Resolution: NLT 5.0 between betamethasone valerate and betamethasone valerate related compound H;
NLT 1.5 between betamethasone valerate related compound H and betamethasone valerate related compound D, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of betamethasone and betamethasone valerate related compound A in the portion of Betamethasone Valerate taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of betamethasone or betamethasone valerate related compound A from the Sample solution
rₛ = peak response of betamethasone or betamethasone valerate related compound A from the Standard solution
Cₛ = concentration of USP Betamethasone RS or USP Betamethasone Valerate Related Compound A RS in the Standard solution (μg/mL)
Cᵤ = concentration of Betamethasone Valerate in the Sample solution (μg/mL)
Calculate the percentage of 9-fluoroprednisolone 17-valerate, betamethasone valerate related compound C, betamethasone valerate related compound D, 6α-bromobetamethasone 17-valerate, and any unspecified impurity in the portion of Betamethasone Valerate taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × (1/F) × 100
rᵤ = peak response of 9-fluoroprednisolone 17-valerate, betamethasone valerate related compound C, betamethasone valerate related compound D, 6α-bromobetamethasone 17-valerate, or any unspecified impurity from the Sample solution
rₛ = peak response of betamethasone valerate from the Standard solution
Cₛ = concentration of USP Betamethasone Valerate RS in the Standard solution (μg/mL)
Cᵤ = concentration of Betamethasone Valerate in the Sample solution (μg/mL)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1. The reporting threshold is 0.05%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Betamethasone | 0.27 | — | 0.7 |
| 9-Fluoroprednisolone 17-valerate (a) | 0.59 | 0.95 | 0.15 |
| Betamethasone valerate related compound C (b) | 0.82 | — | 0.15 |
| Betamethasone valerate | 1.0 | — | — |
| Betamethasone valerate related compound H (c,d) | 1.3 | — | — |
| Betamethasone valerate related compound D (e) | 1.4 | 1.0 | 0.10 |
| Betamethasone valerate related compound A (f) | 1.6 | — | 0.5 |
| 6α-Bromobetamethasone 17-valerate (g) | 1.9 | 0.83 | 0.3 |
| Any unspecified impurity | — | 1.0 | 0.1 |
| Total impurities (h) | — | — | 1.5 |
a. 9-Fluoro-11β,21-dihydroxy-3,20-dioxopregna-1,4-diene-17-yl valerate.
b. 9-Fluoro-11β,21-dihydroxy-16α-methyl-3,20-dioxopregna-1,4-dien-17-yl valerate; also known as Dexamethasone 17-valerate.
c. Also known as beclomethasone 17-valerate.
d. This impurity is quantified using the Limit of Betamethasone Valerate Related Compound H test.
e. Also known as 9α-bromobetamethasone 17-valerate.
f. Also known as betamethasone 21-valerate.
g. 6α-Bromo-9-fluoro-11β,21-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17-yl valerate.
h. The sum of all impurities from the Organic Impurities and the Limit of Betamethasone Valerate Related Compound H tests.
(USP 1-Aug-2024)
5 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 10 mg/mL in dioxane
Acceptance criteria: +75° to +82°
Loss on Drying 〈731〉
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Betamethasone RS (USP 1-Aug-2024)
USP Betamethasone Valerate RS
USP Betamethasone Valerate Related Compound A RS
9-Fluoro-11β,17-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-21-yl valerate.
C₂₇H₃₇FO₆ 476.59
USP Betamethasone Valerate Related Compound D RS
9-Bromo-11β,21-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-dien-17-yl valerate.
C₂₇H₃₇BrO₆ 537.49
USP Betamethasone Valerate System Suitability Mixture RS
It contains a mixture of the following 2 compounds:
Betamethasone valerate
Betamethasone valerate related compound H: 9-Chloro-11β,21-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17-yl valerate.
C₂₇H₃₇ClO₆ 493.04 (USP 1-Aug-2024)


