Betamethasone Sodium Phosphate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
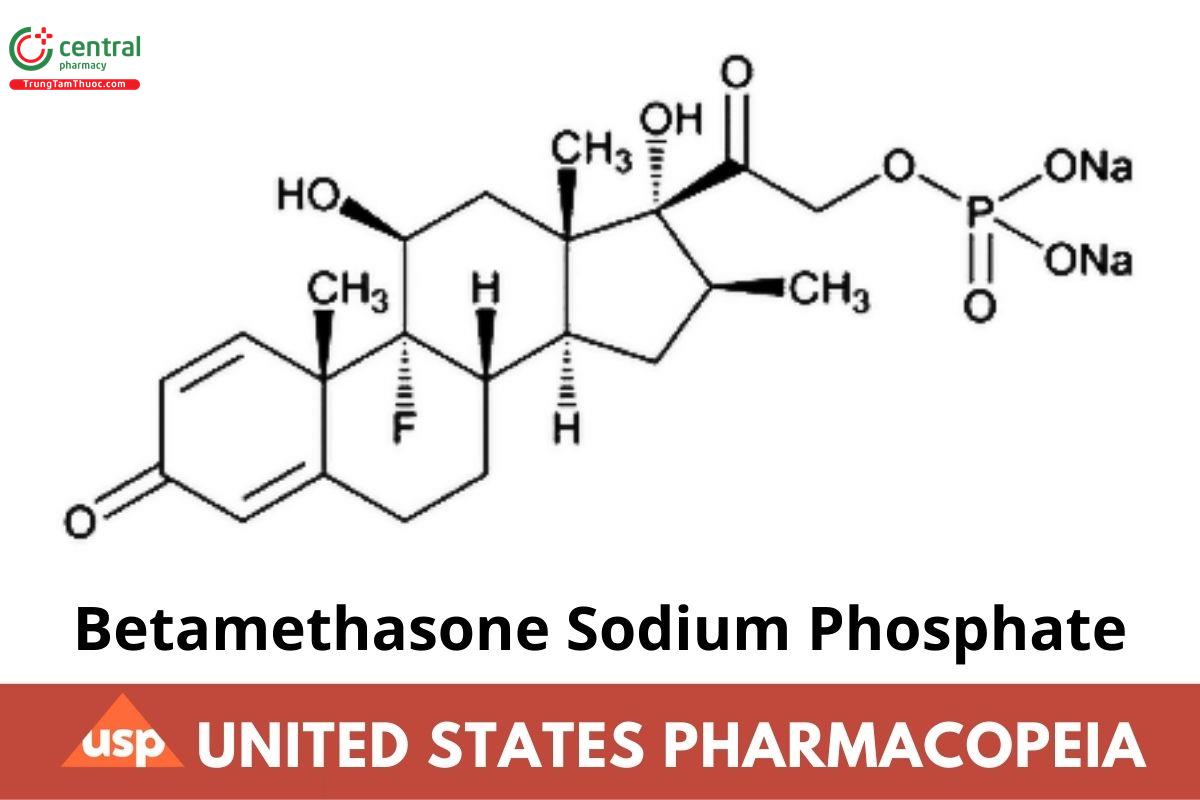
C22H28FNa2O8P 516.40
Pregna-1,4-diene-3,20-dione, 9-uoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, disodium salt, (11β,16β)-; 9-Fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 21-(disodium phosphate) CAS RN: 151-73-5; UNII: 7BK02SCL3W
1 DEFINITION
Betamethasone Sodium Phosphate contains NLT 97.0% and NMT 103.0% of betamethasone sodium phosphate (C22H28FNa2O8P), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M (CN 1-May-2020)
B. THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST (201)
Standard solution: 1 mg/mL of USP Betamethasone Sodium Phosphate RS in methanol
Sample solution: 1 mg/mL of Betamethasone Sodium Phosphate in methanol
Chromatographic system
Application volume: 10 µL
Developing solvent system: 500 mL of butyl alcohol and 200 mL of dilute hydrochloric acid (1 in 12). Place in a separatory funnel, and mix.
Use the organic layer as the developing solvent.
Spray reagent: Sulfuric acid, methanol, and nitric acid (10:10:1)
Analysis
Samples: Standard solution and Sample solution
Proceed as directed in the chapter, except to spray the plate with Spray reagent, and heat at 105° for 10 min.
Acceptance criteria: Meets the requirements
C. IDENTIFICATION TESTS GENERAL, Sodium (191) and Phosphate (191)
Analysis: Ignite it at 800° (see Residue on Ignition (281)).
Acceptance criteria: The residue meets the requirements for sodium and phosphate.
3 ASSAY
PROCEDURE
Mobile phase: Methanol and 0.07 M anhydrous monobasic potassium phosphate (3:2)
Diluent: Methanol and water (3:2)
Standard solution: 0.17 mg/mL of USP Betamethasone Sodium Phosphate RS in Diluent
Sample solution: 0.17 mg/mL of Betamethasone Sodium Phosphate in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm x 30-cm; packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2
Relative standard deviation: NMT 3.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of betamethasone sodium phosphate (C22H28FNa2O8P) in the portion of Betamethasone Sodium Phosphate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Betamethasone Sodium Phosphate RS in the Standard solution (mg/mL)
Cu = concentration of Betamethasone Sodium Phosphate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the anhydrous basis
4 IMPURITIES
4.1 LIMIT OF PHOSPHATE IONS
Standard phosphate solution and Phosphate reagent A: Prepare as directed in Phosphate in Reagents (see Reagents, Indicators, and
Solutions-General Tests for Reagents).
Phosphate reagent B: Dissolve 350 mg of p-methylaminophenol sulfate in 50 mL of water. Add 20 g of sodium metabisulfite, mix to dissolve, and dilute with water to 100 mL
Standard solution: Dilute 5.0 mL of Standard phosphate solution in a mixture of 10 mL of water and 5 mL of 2 N sulfuric acid contained in a 25-mL volumetric flask, by warming if necessary. Add 1 mL each of Phosphate reagent A and Phosphate reagent B, dilute with water to 25.0 mL, mix, and allow to stand at room temperature for 30 min.
Sample solution: Dissolve 50 mg of Betamethasone Sodium Phosphate in a mixture of 10 mL of water and 5 mL of 2 N sulfuric acid contained in a 25-mL volumetric flask, by warming if necessary. Add 1 mL each of Phosphate reagent A and Phosphate reagent B, dilute with water to 25.0 mL, mix, and allow to stand at room temperature for 30 min.
Instrumental conditions
Mode: Vis
Analytical wavelength: Maximum absorbance at about 730 nm
Cell: 1 cm
Blank: Water
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution. The limit is 1.0% of phosphate (PO4).
4.2 LIMIT OF FREE BETAMETHASONE
Sample stock solution: 1.0 mg/mL of Betamethasone Sodium Phosphate in water, prepared as follows. Dissolve 25.0 mg of Betamethasone Sodium Phosphate in water to make 25.0 mL.
Sample solution: Transfer 5.0 mL of the Sample stock solution to a separator, and extract with three 25-ml portions of chloroform. Filter each extract through a chloroform-saturated cotton pledget, combining the filtrates in a conical flask. Evaporate the chloroform on a steam bath to dryness with the aid of a current of air, and dissolve the residue in methanol to make 25.0 mL.
Blank solution: Transfer 5.0 mL of water to a separator. Proceed as directed in Sample solution, beginning with "extract with three 25-mL portions of chloroform".
Instrumental conditions
Mode: UV
Analytical wavelength: Maximum absorbance at about 239 nm
Cell: 1 cm
Blank: Blank solution
Analysis
Sample: Sample solution
Calculate the quantity, in mg, of free betamethasone in the portion of Betamethasone Sodium Phosphate taken:
Result = A × 3.125
A = absorbance of the Sample solution
Acceptance criteria: NMT 0.25 mg (1.0%)
5 SPECIFIC TESTS
OPTICAL ROTATION, Specific Rotation(781S)
Sample solution: 10 mg/mL
Acceptance criteria: +99° to +105°
WATER DETERMINATION, Method (921): NMT 10.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11)
USP Betamethasone Sodium Phosphate RS


