Betamethasone Dipropionate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
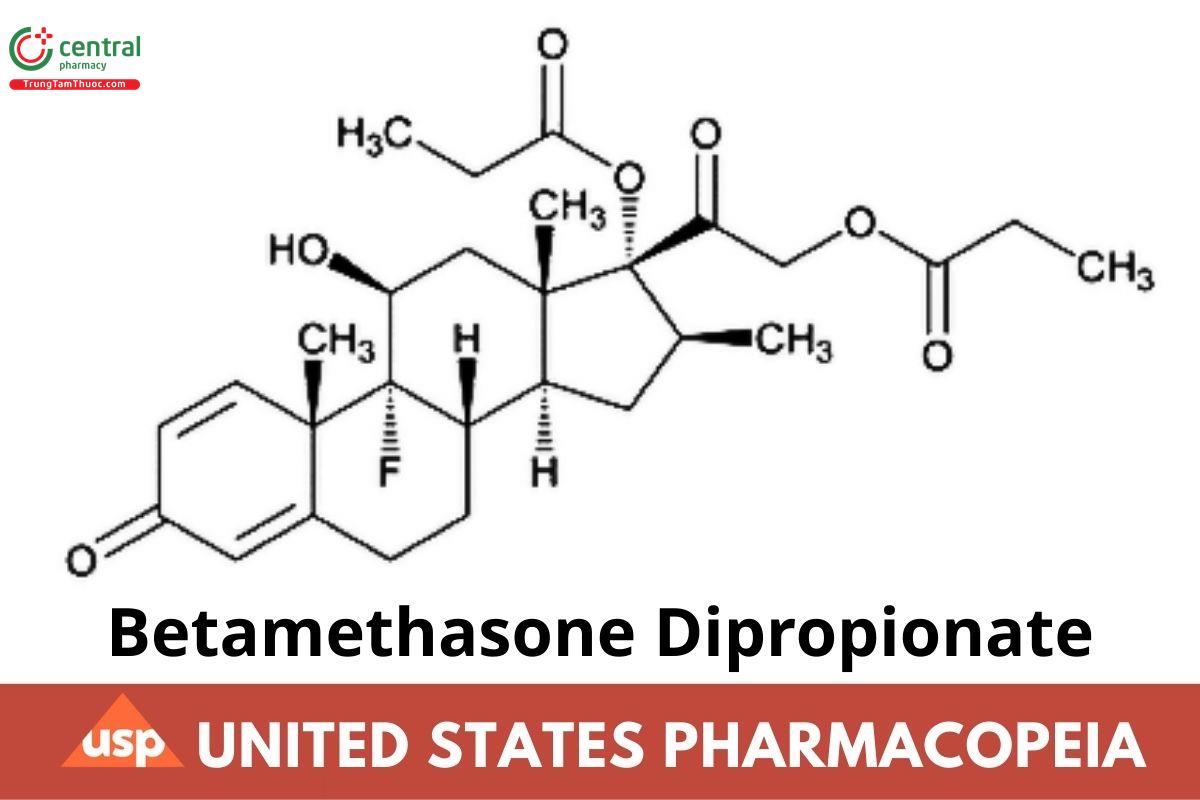
C28H37FO7 504.59
Pregna-1,4-diene-3,20-dione, 9-uoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11β,16β);
9-Fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate CAS RN: 5593-20-4; UNII: 826Y60901U.
1 DEFINITION
Betamethasone Dipropionate contains NLT 97.0% and NMT 103.0% of betamethasone dipropionate (C28H37FO7), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M ( CN 1-Mar-2020)
B. THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST (201)
Sample solution: 1 mg/ml, in chloroform
Chromatographic system
Developing solvent system: Chloroform and acetone (7:1)
Acceptance criteria: Meets the requirements
3 ASSAY
PROCEDURE
Mobile phase: Acetonitrile and water (1 in 2) such that the retention times for betamethasone dipropionate and beclomethasone dipropionate are 14 and 18 min, respectively. Degas by sonicating for 5-10 min. Do not leave Mobile phase in the column overnight, but flush the system after use with water for 15 min, followed by methanol for 15 min.
Diluent: Acetic acid and methanol (1 in 1000)
Internal standard solution: 0.9 mg/mL of USP Beclomethasone Dipropionate RS in Diluent
Standard stock solution: 0.6 mg/mL of USP Betamethasone Dipropionate RS in Diluent
Standard solution: 0.3 mg/mL of USP Betamethasone Dipropionate RS and 0.45 mg/mL of USP Beclomethasone Dipropionate RS, prepared by combining 5.0 ml each of the Internal standard solution and the Standard stock solution
Sample stock solution: 0.6 mg/mL of Betamethasone Dipropionate in Diluent
Sample solution: 0.3 mg/mL of Betamethasone Dipropionate, prepared by combining 5.0 mL each of the Internal standard solution and the Sample stock solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 or 240 nm
Column: 4-mm x 30-cm; packing L1
Injection volume: 5-25 µL
System suitability
Sample: Standard solution
Suitability requirements
Peak area ratios: The lowest and highest peak area ratios of three successive injections agree within 2.0%.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of betamethasone dipropionate (C28H37FO7) in the portion of Betamethasone Dipropionate taken:
Result = (Ru /Rs ) × (Cs /Cu ) × 100
Ru = peak height ratio of betamethasone dipropionate to the internal standard from the Sample solution
Rs = peak height ratio of betamethasone dipropionate to the internal standard from the Standard solution
Cs = concentration of USP Betamethasone Dipropionate RS in the Standard solution (mg/mL)
Cu = concentration of Betamethasone Dipropionate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the dried basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.2%, using a platinum crucible
ORGANIC IMPURITIES
Mobile phase: Acetonitrile and water (65:35)
System suitability solution: 0.05 mg/mL of USP Betamethasone Dipropionate RS and 0.05 mg/mL of USP Betamethasone Valerate RS in
Mobile phase
Sample solution: 0.3 mg/mL of Betamethasone Dipropionate in Mobile phase. Shake until dissolved.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 15-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 4.0 between betamethasone valerate and betamethasone dipropionate
Column efficiency: NLT 8000 theoretical plates
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Betamethasone Dipropionate taken:
Result = (ru /rT ) × 100
ru = peak response for each impurity
rT = sum of the responses for all the peaks
Acceptance criteria
Individual impurities: NMT 1.0%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
OPTICAL ROTATION, Specific Rotation(781S)
Sample solution: 10 mg/mL, in dioxane
Acceptance criteria: +63° to +70°
LOSS ON DRYING (731)
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers. Store at 25°, excursions permitted between 15° and 30°.
USP REFERENCE STANDARDS (11)
USP Beclomethasone Dipropionate RS
USP Betamethasone Dipropionate RS
USP Betamethasone Valerate RS


