Bendamustine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
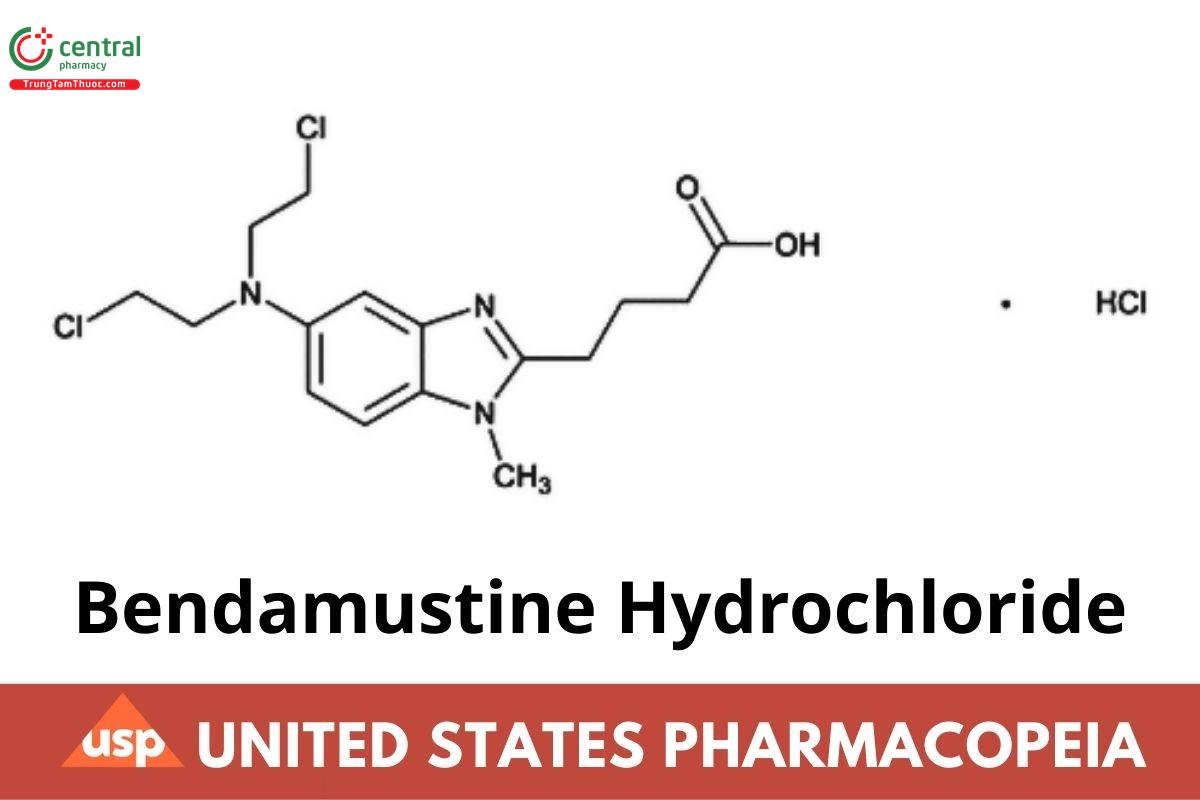
C16H21CI2N3O2 . HCI 394.72
1H-Benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-1-methyl-, monohydrochloride;
4-(5-[Bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazole-2-yl)butanoic acid monohydrochloride CAS RN®: 3543-75-7.
Bendamustine (free base)
C16H21CI2N3O2 358.26 CAS RN®: 16506-27-7.
Monohydrate
C16H21CI2N3O2 . HCI . H2O 412.74 CAS RN®: 1374784-02-7.
1 DEFINITION
Bendamustine Hydrochloride is anhydrous or contains one molecule of hydration. The anhydrous form contains NLT 98.0% and NMT 102.0% of bendamustine hydrochloride (C16H21CI2N3O2 . HCI), calculated on the as-is basis. The monohydrate form contains NLT 98.0% and NMT 102.0% of bendamustine hydrochloride (C16H21CI2N3O2 . HCI), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. ASPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A or 197K (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. IDENTIFICATION TESTS-GENERAL (191), Chemical Identification Tests, Chloride
3 ASSAY
3.1 PROCEDURE
Solution A: 0.1% (v/v) trifluoroacetic acid in water
Solution B: 0.1% (v/v) trifluoroacetic acid in acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 93 | 7 |
| 5 | 93 | 7 |
| 13 | 73 | 27 |
| 16 | 73 | 27 |
| 25 | 43 | 57 |
| 26 | 10 | 90 |
| 31 | 10 | 90 |
| 40 | 93 | 7 |
| 45 | 93 | 7 |
Diluent: 1-Methyl-2-pyrrolidone and Solution A (1:1)
Standard solution: 4.2 mg/mL of USP Bendamustine Hydrochloride RS in Diluent
Sample solution: 4.2 mg/mL of Bendamustine Hydrochloride in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L60
Temperatures
Autosampler: 2°-8°
Column: 30°
Flow rate: 1 mL/min
Injection volume: 2 µL
Analysis time: 25 min
System suitability
[NOTE-The slower syringe draw rate and higher detector sampling rate can be applied in order to improve the precision.]
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of bendamustine hydrochloride (C16H21CI2N3O2 . HCI) in the portion of Bendamustine Hydrochloride taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution s
CS = concentration of USP Bendamustine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Bendamustine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the as-is basis for the anhydrous form; 98.0%-102.0% on the anhydrous and solvent-free basis for the monohydrate form
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281): NMT 0.1%
Change to read:
4.2 ORGANIC IMPURITIES
Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability solution: 4.2 mg/mL of USP Bendamustine Hydrochloride RS, and 0.02 mg/mL each of USP Bendamustine Related Compound A RS, USP Bendamustine Related Compound C RS, USP Bendamustine Related Compound D RS, USP Bendamustine Related Compound E RS, USP Bendamustine Related Compound G RS, USP Bendamustine Related Compound H RS, and USP Bendamustine Related Compound I RS in Diluent
Sensitivity solution: 2 µg/mL of USP Bendamustine Hydrochloride RS in Diluent, from the Standard solution
System suitability
Samples: System suitability solution and Sensitivity solution
Suitability requirements
Resolution: NLT 5 between the bendamustine related compound G and bendamustine peaks; NLT 4 between the bendamustine related compound H and bendamustine related compound I peaks, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Bendamustine Hydrochloride taken:
Result = (r /{Σ[rU × (1/F)] + rS}) × (1/F) × 100
rU = peak area of each impurity from the Sample solution
F = relative response factor for each impurity (see Table 2)
rS = peak area of bendamustine from the Sample solution
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
Bendamustine related compound A | 0.25 | 0.76 | 0.25 |
| Bendamustine related compound C | 0.60 | 0.83 | 0.20 |
| Bendamustine related compound D | 0.69 | 0.93 | 0.15 (RB 1-Mar-2019) |
| Bendamustine related compound E | 0.73 | 1.2 | 0.45 |
| Bendamustine related compound G | 0.90 | 3.1 | 0.35 |
| Bendamustine | 1.0 | - | - |
| Bendamustine related compound H | 1.15 | 0.98 | 0.30 |
| Bendamustine related compound I | 1.20 | 1.1 | 0.40 |
| Any individual unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 1.0 |
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method I, Method la: NMT 1.0% for the anhydrous form; 3.0%-5.5% for the monohydrate form
BACTERIAL ENDOTOXINS TEST (85): Meets the requirements
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62): The total aerobic microbial count is NMT 103 cfu/g. The total
combined molds and yeasts count is NMT 102 cfu/g.
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in well-closed containers. Store at room temperature.
6.2 USP REFERENCE STANDARDS (11)
USP Bendamustine Hydrochloride RS
USP Bendamustine Related Compound A RS
4-(5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl)butanoic acid.
C16H23N3O4 321.38
USP Bendamustine Related Compound C RS
Ethyl 4-(5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl)butanoate.
C18H27N3O4 349.43
USP Bendamustine Related Compound D RS
4-(5-[(2-Chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl)butanoic acid.
C14H18CIN3O2 295.77
USP Bendamustine Related Compound E RS
4-{5-[(2-Chloroethyl) (2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoic acid.
C16H22CIN3O3 339.82
USP Bendamustine Related Compound G RS
4-[6-(2-Chloroethyl)-3,6,7,8-tetrahydro-3-methylimidazo [4,5-h] [1,4]benzothiazin-2-yl]butanoic acid.
C16H20CIN3O2S 353.86
USP Bendamustine Related Compound H RS
4-[5-((2-[(4-(5-[Bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoyl)oxy]ethyl)(2-chloroethyl)amino)-1-methyl-1H-benzimidazol-2-yl]butanoic acid.
C32H41CI3N6O4 680.07
USP Bendamustine Related Compound I RS
Ethyl 4-(5-[bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl)butanoate.
C18H25CI2N3O2 386.32


