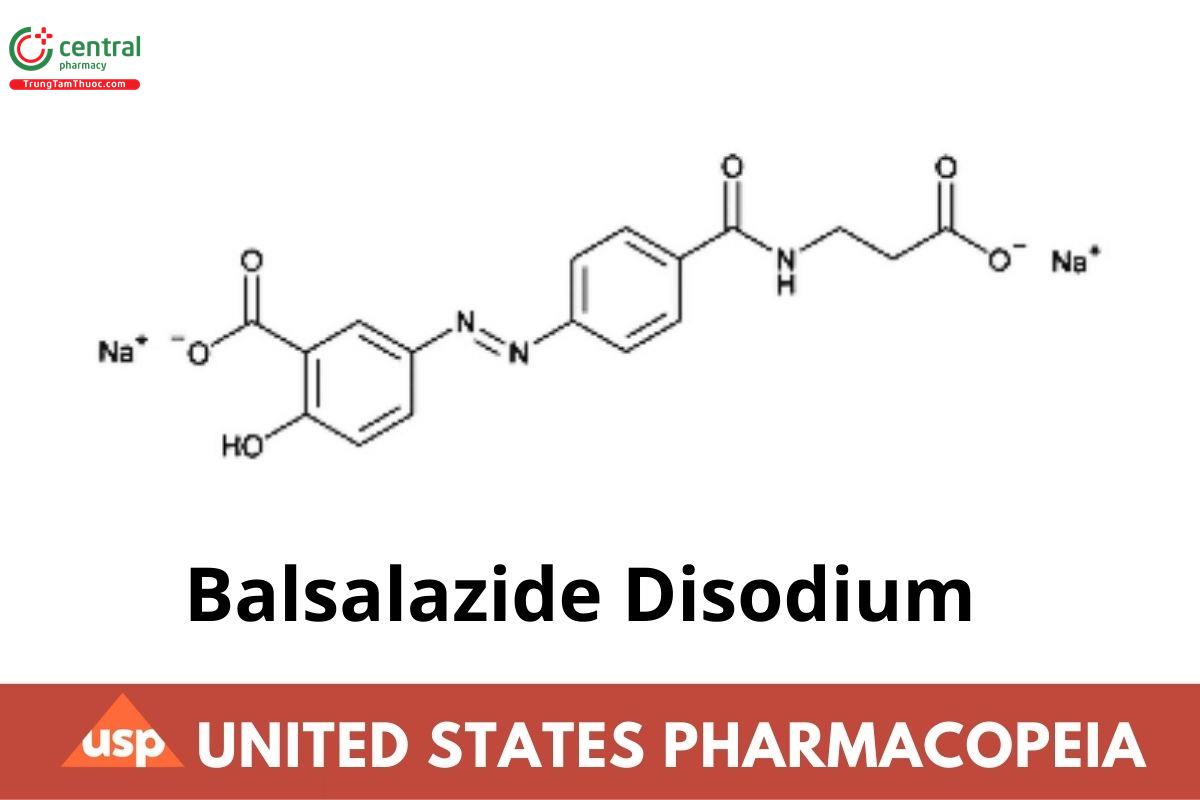
Balsalazide Disodium
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Balsalazide Disodium contains NLT 98.0% and NMT 102.0% of C₁₇H₁₃N₃Na₂O₆·2H₂O, calculated on the as-is basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Sample solution: 10 µg/mL in water
C. Identification Tests—General, Sodium 〈191〉
3 ASSAY
Procedure
Sample: 219 mg
Analysis: Add 80 mL of glacial acetic acid to the Sample, sonicate to dissolve, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction (see Titrimetry 〈541〉). Each mL of 0.1 N perchloric acid is equivalent to 21.87 mg of C₁₇H₁₃N₃Na₂O₆·2H₂O.
Acceptance criteria: 98.0%–102.0% on the as-is basis
4 IMPURITIES
Organic Impurities
[Note—On the basis of the synthetic route, perform either Procedure 1 or Procedure 2. Procedure 2 is recommended when impurities 1, 2, and 3 listed in Impurity Table 2 may be present.]
Procedure 1
Solution A: Dissolve 2.7 g of monobasic potassium phosphate in 1000 mL of water, and adjust with 10% potassium hydroxide solution to a pH of 6.00±0.1.
Diluent: Use Solution A.
Solution B: Use acetonitrile.
Standard solution: 0.5 µg/mL of USP Balsalazide Disodium RS, 0.5 µg/mL of USP Balsalazide Related Compound A RS, 0.5 µg/mL of USP Balsalazide Related Compound B RS, and 0.5 µg/mL of USP Salicylic Acid RS in Diluent. If needed, a small amount of acetonitrile may be added to facilitate dissolution.
Sample solution: 1 mg/mL of Balsalazide Disodium in Diluent
Mobile phase: See the gradient table below.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 4 | 90 | 10 |
| 40 | 75 | 25 |
| 47 | 75 | 25 |
| 55 | 50 | 50 |
| 60 | 50 | 50 |
| 60.1 | 90 | 10 |
| 70 | 90 | 10 |
Chromatographic system
Mode: LC
Detector: UV 238 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 45°
Flow rate: 1 mL/min
Injection size: 30 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 5 between balsalazide and balsalazide related compound B
Relative standard deviation: NMT 5% for each peak
Tailing factor: NMT 1.8 for the balsalazide peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Balsalazide Disodium taken:
Result = (r₍ᵤ₎/r₍ₛ₎) × (C₍ₛ₎/C₍ᵤ₎) × 100
r₍ᵤ₎ = peak response for each individual impurity from the Sample solution
r₍ₛ₎ = peak response for the corresponding impurity from the Standard solution. For unspecified impurities, r₍ₛ₎ is the peak response for the balsalazide peak from the Standard solution.
C₍ₛ₎ = concentration of the corresponding impurity in the Standard solution (mg/mL). For unspecified impurities, C₍ₛ₎ is the concentration of balsalazide disodium in the Standard solution.
C₍ᵤ₎ = concentration of Balsalazide Disodium in the Sample solution (mg/mL)
Acceptance criteria
Individual impurities: See Impurity Table 1.
Reporting level for impurities: 0.03%
Total impurities: NMT 1.0%
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Salicylic acid | 0.37 | 0.05 |
| Balsalazide related compound A¹ | 0.70 | 0.05 |
| Balsalazide | 1.00 | — |
| Balsalazide related compound B² | 1.2 | 0.05 |
| Any other individual unspecified impurity | — | 0.05 |
¹ (E)-5-[(p-Carboxyphenyl)azo]-2-salicylic acid.
² (E)-5-({m-[(2-Carboxyethyl)carbamoyl]phenyl}azo)-2-salicylic acid.
Procedure 2
Solution A: Prepare 50 mM monobasic potassium phosphate buffer as follows: Dissolve 6.8 g of monobasic potassium phosphate in 1000 mL of water, and adjust with 2 N potassium hydroxide solution to a pH of 6.8–7.0.
Solution B: Acetonitrile, methanol and Solution A (5:1:14)
Solution C: Acetonitrile, methanol and Solution A (9:1:10)
Diluent: Use Solution B.
Mobile phase: See the gradient table below.
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
| 0 | 100 | 0 | 0 |
| 37 | 0 | 100 | 0 |
| 60 | 0 | 0 | 100 |
| 75 | 100 | 0 | 0 |
| 85 | 100 | 0 | 0 |
Standard solution: 0.075 mg/mL of USP Balsalazide Disodium RS in Diluent.
Sensitivity solution: 0.375 µg/mL in Diluent, from Standard solution
System suitability solution: 1.5 mg/mL of USP Balsalazide Disodium RS and 1.5 µg/mL of USP Balsalazide Related Compound A RS in Diluent
Sample solution: 1.5 mg/mL of Balsalazide Disodium in Diluent.
Chromatographic system
Mode: LC
Detector: UV 300 nm
Column: 4.6-mm × 15-cm; 3-µm packing L1
Column temperature: 25°–27°
Flow rate: 1 mL/min
Injection size: 10 µL
System suitability
Samples: Standard solution, Sensitivity solution, and System suitability solution
Suitability requirements
Resolution: NLT 8.5 between balsalazide related compound A and balsalazide, from the System suitability solution
Tailing factor: NMT 3.4 for the balsalazide peak, from the System suitability solution
Signal-to-noise ratio: NLT 10, from the Sensitivity solution
Relative standard deviation: NMT 2.0%, from the Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Balsalazide Disodium taken:
Result = (r₍ᵤ₎/r₍ₛ₎) × (C₍ₛ₎/C₍ᵤ₎) × (1/F) × 100
r₍ᵤ₎ = peak response for each individual impurity from the Sample solution
r₍ₛ₎ = peak response for balsalazide from the Standard solution
C₍ₛ₎ = concentration of USP Balsalazide Disodium RS in the Standard solution
C₍ᵤ₎ = concentration of Balsalazide Disodium in the Sample solution (mg/mL)
F = relative response factor (see Impurity Table 2)
Acceptance criteria
Individual impurities: See Impurity Table 2.
Reporting level for impurities: 0.03%
Total impurities: NMT 0.50%
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| N-(4-Aminobenzoyl)-β-alanine¹ | 0.29 | 1.8 | 0.05 |
| Salicylic acid | 0.55 | 1.4 | 0.05 |
| Balsalazide related compound A² | 0.88 | 1.4 | 0.05 |
| Impurity 1³ | 0.91 | 1.9 | 0.05 |
| Impurity 2⁴ | 0.92 | 1.4 | 0.05 |
| Impurity 3⁵ | 0.94 | 0.83 | 0.05 |
| Balsalazide | 1.00 | — | — |
| Impurity 4⁶ | 1.35 | 2.1 | 0.05 |
| Impurity 5⁷ | 1.77 | 0.91 | 0.05 |
| Any other individual unspecified impurity | — | — | 0.05 |
¹ This impurity may be present as two peaks. Use the sum of the two peaks to determine compliance.
² (E)-5-[(p-Carboxyphenyl)azo]-2-salicylic acid.
³ (E,E)-3,5-di-[4-(2-Carboxyethylcarbamoyl) phenylazo]-salicylic acid.
⁴ (E)-3-[4-(2-Carboxyethylcarbamoyl) phenylazo]-salicylic acid.
⁵ (E,E)-5-{{2-[4-(2-Carboxyethylcarbamoyl)phenylazo]-4-[2-carboxyethyl-carbamoyl]}phenylazo}-salicylic acid.
⁶ (E)-2-[4-(2-Carboxyethylcarbamoyl)phenoxy]-5-{[4-(2-carboxyethyl-carbamoyl)]phenylazo}-benzoic acid
⁷ (E)-2-Hydroxy-5-[[4-[[(3-isopropoxy-3-oxopropyl)amino]carbonyl]phenyl]azo]benzoic acid
5 SPECIFIC TESTS
Water Determination, Method Ia 〈921〉: 7.8%–9.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
Labeling: If a test for Organic Impurities other than Test 1 is used, the labeling states the test with which the article complies.
Change to read:
USP Reference Standards 〈11〉
USP Balsalazide Disodium RS
USP Balsalazide Related Compound A RS
(E)-5-[(p-Carboxyphenyl)azo]-2-salicylic acid, disodium salt. C₁₄H₈N₂O₅Na₂ ▲330.21▲ (CN 1-Dec-2024)
USP Balsalazide Related Compound B RS
(E)-5-({m-[(2-Carboxyethyl)carbamoyl]phenyl}azo)-2-salicylic acid. C₁₇H₁₅N₃O₆ ▲357.32▲ (CN 1-Dec-2024)
USP Salicylic Acid RS


