Aztreonam
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
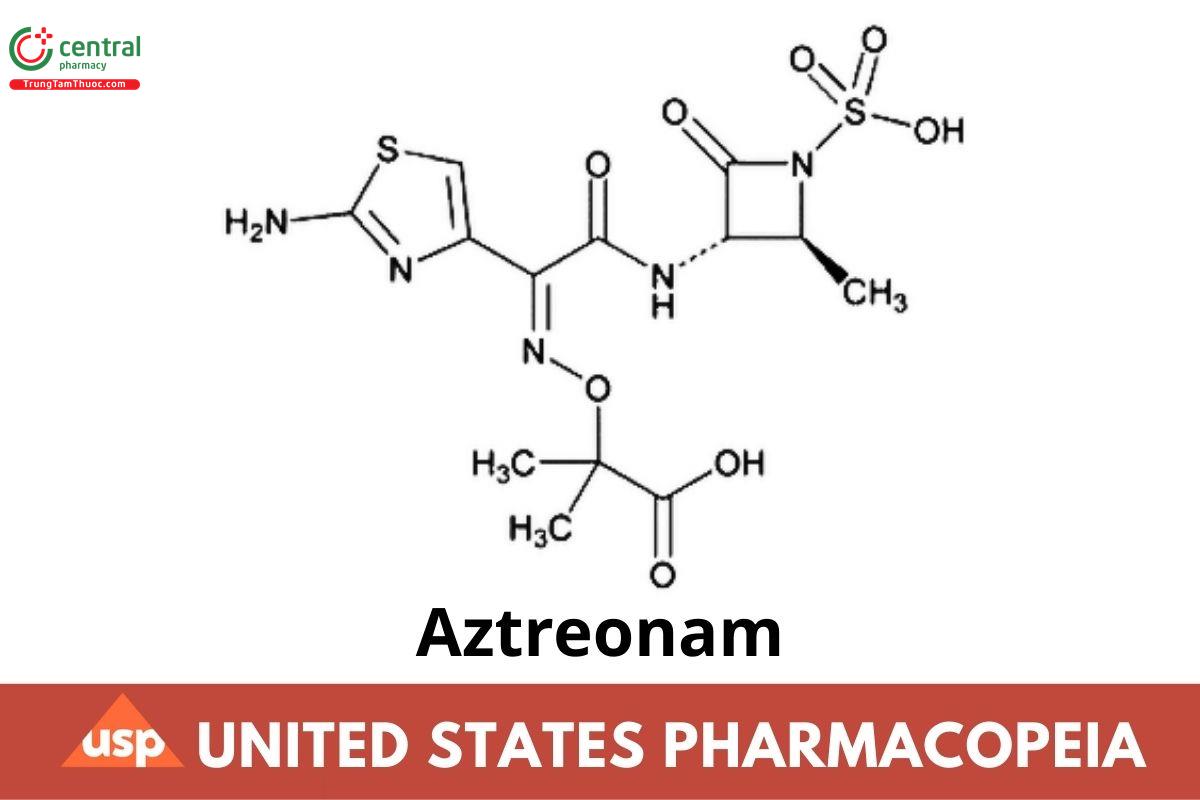
C13H17N5O8S2 435.43
Propanoic acid, 2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methyl-, [2S-[2α,3β(Z)]]-;(Z)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid CAS RN: 78110-38-0; UNII: G2B4VE5GH8.
1 DEFINITION
Aztreonam, which may be anhydrous or hydrated, contains NLT 92.0% and NMT 105.0% of aztreonam (C13H17N5O8S2), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020): If a difference appears in the IR spectra of the analyte and the standard, dissolve equal portions of the test specimen and the Reference Standard in equal volumes of methanol. [NOTE-TO achieve a complete dissolution, it is suggested to use about 25 ml of methanol for each 50 mg of material, and stir the mixture for 40 min at room temperature.]
Evaporate the solutions to dryness under vacuum, and dry at 40 for 4 h under vacuum. Perform the test on the residues.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
[NOTE-Store the System suitability solution, Standard solution, and Sample solution at 5o, and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer.]
Buffer: 6.8 mg/mL of monobasic potassium phosphate in water. Adjust with 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Methanol and Buffer (1:4)
System suitability solution: 1 mg/mL of USP Aztreonam RS and 1 mg/mL of USP Aztreonam E-Isomer RS in Mobile phase
Standard solution: 1 mg/ml. of USP Aztreonam RS in Mobile phase
Sample solution: 1 mg/mL of Aztreonam in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm x 30-cm; 10-µm packing 11
Flow rate: 1.5 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for aztreonam and aztreonam E-isomer are 1.0 and 1.8, respectively.]
Suitability requirements
Resolution: NLT 2.0 between aztreonam and aztreonam E-isomer, System suitability solution
Tailing factor: NMT 2 for aztreonam, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of aztreonam (C13H17N5O8S2) in the portion of Aztreonam taken:
Result = (ru /rs ) × (Cs /Cu ) × P × F × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Aztreonam RS in the Standard solution (mg/mL)
Cu = concentration of Aztreonam in the Sample solution (mg/mL)
P = potency of USP Aztreonam RS (µg/mg)
F = unit conversion factor, 0.001 mg/µg
Acceptance criteria: 92.0%–105.0% on the anhydrous and solvent-free basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid
ORGANIC IMPURITIES
[NOTE-Store the System suitability solution, Standard solution, and Sample solution at 5", and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer.]
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay..
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Aztreonam taken:
Result = (ru /rs ) × (Cs /Cu ) × P × F × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of aztreonam from the Standard solution
Cs = concentration of USP Aztreonam RS in the Standard solution (mg/mL)
Cu = concentration of Aztreonam in the Sample solution (mg/mL)
P = potency of USP Aztreonam RS (µg/mg)
F = unit conversion factor, 0.001 mg/µg
Acceptance criteria: See Table 1.
Table 1
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Open-ring aztreonama and open-ring desulfated aztreonamb,c | 0.55 | 1.0 |
| Aztreonam (Z-isomer) | 1.0 | - |
| Desulfated aztreonamd | 1.6 | 1.5 |
| Aztreonam E-isomere | 1.8 | 0.5 |
| Aztreonam ethyl esterf | 3.9 | 1.5 |
| Any individual unspecied impurity | - | 0.1 |
| Total impurities | - | 3.0 |
a (2S,3S)-2-{(Z)-2-[2-Aminothiazol-4-yl]-2-[2-carboxypropan-2-yloxyimino]acetamido}-3-(sulfoamino)butanoic acid.
b (2S,3S)-3-Amino-2-{(Z)-2-[2-aminothiazol-4-yl]-2-[2-carboxypropan-2-yloxyimino]acetamido}butanoic acid.
c Open-ring aztreonam and open-ring desulfated aztreonam coelute. The limit is for the sum of these two impurities.
d (Z)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
e (E)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
f Ethyl (Z)-2-({[(2-amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionate.
5 SPECIFIC TESTS
STERILITY TESTS (71), Test for Sterility of the Product to Be Examined, Membrane Filtration: Where the label states that Aztreonam is sterile, it meets the requirements using Fluid A, to which 23.4 g of sterile Arginine has been added to each 1000 ml..
WATER DETERMINATION (921), Method /: NMT 2.0%; if labeled as the hydrated form: 12.0%~18.0%. (NOTE-The term "hydrated form" refers to the a form of Aztreonam, which is not a stoichiometric hydrate.]
BACTERIAL ENDOTOXINS TEST (85): Where the label states that Aztreonam is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.17 USP Endotoxin Units/mg of aztreonam.
LIMIT OF ALCOHOL
[NOTE-This test is to be performed if alcohol is used while manufacturing Aztreonam.]
Standard solution: 0.004 mL/mL of alcohol from USP Alcohol Determination+Alcohol RS and 0.004 mL/mL of acetonitrile from USP Alcohol
Determination-Acetonitrile RS in dimethylformamide. [NOTE-The Standard solution contains 0.4% alcohol and 0.4% acetonitrile.]
Sample solution: 80 mg/mL of Aztreonam and 0.004 mL/mL of acetonitrile in dimethylformamide. [NOTE-Dissolve Aztreonam in dimethylformamide using 20% of the final volume. Add a suitable aliquot of USP Alcohol Determination-Acetonitrile RS, and dilute with dimethylformamide to volume. The concentration of acetonitrile in the Sample solution is 0.4%.]
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 3.53 - mm x 30 - m phase G43
Film thickness: 3.0 µm
Temperatures
Injector: 210°
Detector: 280°
Column: See Table 2.
Table 2
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 50 | 0 | 50 | 5 |
| 50 | 10 | 200 | 4 |
Carrier gas: Helium
Linear velocity: 35 cm/s
Injection mode: Split
Injection volume: 0.5 µL
Injection type: Split ratio, 5:1
System suitability
Sample: Standard solution
[Note—The relative retention times for alcohol and acetonitrile are 1.0 and 1.3, respectively.]
Suitability requirements
Resolution: NLT 2.0 between alcohol and acetonitrile
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of alcohol in the portion of Aztreonam taken:
Result = (Ru /Rs ) × [Cs × (D/Cu )] × F × 100
Rs = peak response ratio of alcohol to acetonitrile from the Sample solution
Rs = peak response ratio of alcohol to acetonitrile from the Standard solution
Cs = concentration of alcohol in the Standard solution (mL/mL)
D = density of alcohol (g/mL)
Cu = concentration of Aztreonam in the Sample solution (mg/mL)
F = unit conversion factor, 1000 mg/g
Acceptance criteria: NMT 4%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
LABELING: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms. Where it is the hydrated form, the label so indicates.
USP REFERENCE STANDARDS (11)
USP Alcohol Determination-Acetonitrile RS C2H3N 41.05
USP Alcohol Determinationt Alcohol RS C2H5OH 46.07
USP Aztreonam RS
USP Aztreonam E-lsomer RS
(E)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl carbamoyl methylene]amino}oxy)-2-methylpropionic acid.
C13H17N5O8S2 435.43


