Atorvastatin Calcium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
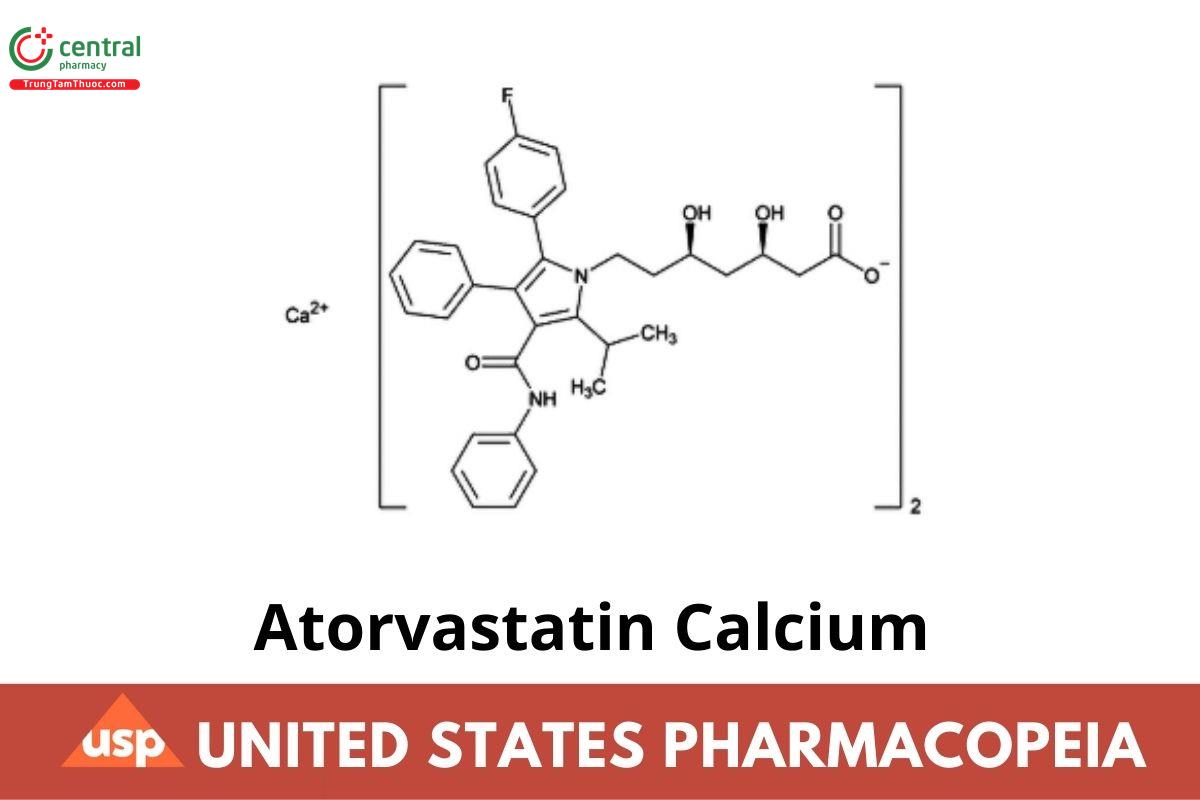
Atorvastatin Calcium
C₆₆H₆₈CaF₂N₄O₁₀ 1155.36
1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, calcium salt (2:1), [R-(R*,R*)]-;
Calcium (βR,δR)-2-(p-fluorophenyl)-β,δ-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoate (1:2);
[(3R,5R)-7-[3-(Phenylcarbamoyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid, calcium salt]
Anhydrous CAS RN®: 134523-03-8; UNII: C0GEJ5QCSO
C₆₆H₆₈CaF₂N₄O₁₀·3H₂O 1209.41
Trihydrate CAS RN®: 344423-98-9; UNII: 48A5M73Z4Q
C₆₆H₆₈CaF₂N₄O₁₀·C₃H₈O₂
Propylene glycol solvate: 1231.46
1 DEFINITION
Atorvastatin Calcium contains NLT 98.0% and NMT 102.0% of atorvastatin calcium (C₆₆H₆₈CaF₂N₄O₁₀), calculated on the anhydrous and solvent-free basis. If labeled as a propylene glycol solvate, it contains NLT 98.0% and NMT 102.0% of atorvastatin calcium (C₆₆H₆₈CaF₂N₄O₁₀), calculated on the anhydrous, propylene glycol-free, and solvent-free basis. It may contain a suitable antioxidant.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
[Note—If a difference appears in the IR spectra of the analyte and the standard, separately dissolve equal portions of the sample specimen and the USP Reference Standard in equal volumes of methanol, evaporate the solution to dryness in similar containers under identical conditions, and repeat the test on the residues.]
B. Calcium
Diluent: Methanol, water, and hydrochloric acid (75:25:2)
Sample solution: 0.05 mg/mL of Atorvastatin Calcium in Diluent
Blank: Diluent
Analysis
Samples: Sample solution and Blank
Instrumental conditions (See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: Calcium emission line at 422.7 nm
Flame: Air–acetylene
Acceptance criteria: The Sample solution exhibits a significant absorption at the calcium emission line at 422.7 nm.
3 ASSAY
Procedure
Buffer: 3.9 g/L of ammonium acetate in water. Adjust with glacial acetic acid to a pH of 5.0 ± 0.1.
Solution A: Acetonitrile, stabilizer-free tetrahydrofuran, and Buffer (21:12:67)
Solution B: Acetonitrile, stabilizer-free tetrahydrofuran, and Buffer (61:12:27)
Mobile phase: See Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 40 | 100 | 0 |
| 70 | 20 | 80 |
| 85 | 0 | 100 |
| 100 | 0 | 100 |
| 105 | 100 | 0 |
| 115 | 100 | 0 |
Diluent: N,N-dimethylformamide
System suitability solution: 0.05 mg/mL of USP Atorvastatin Calcium RS and 0.06 mg/mL of USP Atorvastatin Related Compound B RS in Diluent
Standard solution: 0.4 mg/mL of USP Atorvastatin Calcium RS in Diluent. [Use sonication if necessary.]
Sample solution: 0.4 mg/mL of Atorvastatin Calcium in Diluent. [Use sonication if necessary.]
Chromatographic system (See Chromatography 〈621〉, System Suitability.)
[If significant fronting of the peaks for atorvastatin related compound B and atorvastatin is observed, use the following diluent to prepare the Sample solution, the Standard solution, and the System suitability solution: acetonitrile, stabilizer-free tetrahydrofuran, and water (1:1:2).]
Mode: LC
Detector: UV 244 nm
Column: 4.6-mm × 25-cm; 5-µm packing L7
Column temperature: 35°C
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements:
Resolution: NLT 1.5 between the peaks for atorvastatin related compound B and atorvastatin, System suitability solution
Tailing factor: NMT 1.6, Standard solution
Relative standard deviation: NMT 0.6%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of atorvastatin calcium (C₆₆H₆₈CaF₂N₄O₁₀) in the portion of Atorvastatin Calcium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response from the Sample solution
rs= peak response from the Standard solution
Cs = concentration of USP Atorvastatin Calcium RS in the Standard solution (mg/mL)
Cu = concentration of Atorvastatin Calcium in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous and solvent-free basis. If labeled as a propylene glycol solvate, 98.0%–102.0% on the anhydrous, propylene glycol-free, and solvent-free basis.
4 OTHER COMPONENTS
Content of Propylene Glycol (if labeled as a propylene glycol solvate)
Diluent: Dimethylsulfoxide
Standard solution: 0.125 mg/mL of propylene glycol in Diluent
Sample solution: 2.5 mg/mL of Atorvastatin Calcium (as propylene glycol solvate) in Diluent. Use sonication as needed.
Chromatographic system
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 75-m; 3-µm coating of G43
Temperatures:
Injection port: 230°C
Detector: 250°C
Column: See Table 2.
| Initial Temperature (°C) | Temperature Ramp (°C/min) | Final Temperature (°C) | Hold Time at Final Temperature (min) |
| 100 | 0 | 100 | 1 |
| 100 | 10 | 140 | 5 |
| 140 | 30 | 225 | 3 |
Carrier gas: Helium
Flow rate: 6.0 mL/min
Injection volume: 1 µL
Injection type: Splitless, using a suitable inlet liner
System suitability
Sample: Standard solution
Suitability requirements:
Tailing factor: NMT 2.0
Relative standard deviation: NMT 5.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of propylene glycol in the portion of Atorvastatin Calcium as propylene glycol solvate taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of propylene glycol from the Sample solution
rs = peak response of propylene glycol from the Standard solution
Cs = concentration of propylene glycol in the Standard solution (mg/mL)
Cu = concentration of Atorvastatin Calcium (as propylene glycol solvate) in the Sample solution (mg/mL)
Acceptance criteria: 5.4%–7.3%
5 IMPURITIES
Organic Impurities, Procedure 1
[Note—On the basis of the synthetic route or of the solid state nature of the drug substance, perform either
Procedure 1 or Procedure 2. Procedure 2 may be suitable when atorvastatin lactone, atorvastatin epoxy tetrahydrofuran analog, and atorvastatin
acetonide are possible related compounds, and it may be suitable for an amorphous form of the drug substance.]
Buffer, Solution A, Solution B, Mobile phase, Diluent, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 1.5 µg/mL each of USP Atorvastatin Related Compound A RS, USP Atorvastatin Related Compound B RS, USP Atorvastatin Related Compound C RS, and USP Atorvastatin Related Compound D RS in Diluent
Sample solution: 1 mg/mL of Atorvastatin Calcium in Diluent. [Use sonication if necessary.]
System suitability
Sample: System suitability solution
Suitability requirements:
Resolution: NLT 1.5 between the peaks for atorvastatin related compound B and atorvastatin
Analysis
Samples: Standard solution and Sample solution
Chromatograph the Standard solution, and identify the components based on their relative retention times, given in Table 3.
Calculate the percentage of each of the atorvastatin related compounds A, B, C, and D in the portion of Atorvastatin Calcium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of propylene glycol from the Sample solution
rs = peak response of propylene glycol from the Standard solution
Cs = concentration of propylene glycol in the Standard solution (mg/mL)
Cu = concentration of Atorvastatin Calcium (as propylene glycol solvate) in the Sample solution (mg/mL)
Calculate the percentage of any other individual impurity in the portion of Atorvastatin Calcium taken:
Result = (ru/rs) × 100
ru = peak response of any other individual impurity from the Sample solution
rs = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 3. Disregard any peak observed in the blank; the reporting level for impurities is 0.05%
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Atorvastatin related compound Aa | 0.8 | 0.3 |
| Atorvastatin related compound Bb | 0.9 | 0.3 |
| Atorvastatin | 1.0 | — |
| Atorvastatin related compound Cc | 1.2 | 0.3 |
| Atorvastatin related compound Dd,e | 2.1 | 0.2 |
| Any other individual impurity | — | 0.1 |
| Total impuritiesf | — | 1.0 |
a Desfluoro impurity.
b 3S,5R Isomer.
c Difluoro impurity.
d Epoxide impurity.
e Atorvastatin related compound D may undergo a conversion to its cyclic hemiketal, which is a specified impurity listed in Table 5 in Organic Impurities, Procedure 2, as “atorvastatin epoxy tetrahydrofuran analog”. The cyclic hemiketal of atorvastatin related compound D elutes about 1–2 min before atorvastatin related compound D. Use the sum of the areas of the two peaks as a peak response for atorvastatin related compound D in the Standard solution and the Sample solution.
f This total does not include atorvastatin related compound E, as determined in the Enantiomeric Purity test.
Organic Impurities, Procedure 2
Buffer: pH 5.0 mixture of 0.045 M ammonium formate and 0.0045 M ammonium acetate solutions, prepared as follows...
Solution A: Acetonitrile and Buffer (33:67)
Solution B: Acetonitrile
Solution C: Stabilizer-free tetrahydrofuran
Mobile phase: See Table 4.
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
| 0 | 91 | 0 | 9 |
| 15 | 91 | 6 | 3 |
| 20 | 82 | 16 | 2 |
| 25 | 82 | 16 | 2 |
| 50 | 32 | 66 | 2 |
| 55 | 32 | 66 | 2 |
Diluent: Acetonitrile, stabilizer-free tetrahydrofuran, and Buffer (60:5:35)
Peak identification solution: 0.5 mg/mL of USP Atorvastatin Calcium RS and 2.5 µg/mL each of USP Atorvastatin Related Compound A RS, USP Atorvastatin Related Compound B RS, USP Atorvastatin Related Compound H RS, and USP Atorvastatin Related Compound I RS in Diluent
Sample solution: 0.5 mg/mL of Atorvastatin Calcium in Diluent. Use sonication to dissolve.
[The solution is stable for 3 h at room temperature and for 24 h when stored at 2°–8°, protected from light.]
Chromatographic system
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 4-µm packing L11
Temperatures:
Column: 40°C
Autosampler: 4°C
Flow rate: 1.1 mL/min
Injection volume: 15 µL
System suitability
Sample: Peak identification solution
Suitability requirements:
Peak-to-valley ratio: NLT 2 between the peaks for atorvastatin related compound B and atorvastatin
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Atorvastatin Calcium taken:
Result = (ru/rT) × (1/F) × 100
ru = peak response of the impurity from the Sample solution
rT = sum of all the peak responses, each divided by the corresponding value of the relative response factor from Table 5
F = relative response factor for the impurity (see Table 5)
Acceptance criteria: See Table 5. Disregard any peak eluting before 2 min and any peak observed in the blank; the reporting level for impurities is 0.05%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Atorvastatin diaminoa | 0.58 | 0.74 | 0.15 |
| Atorvastatin related compound Ab | 0.86 | 1.0 | 0.3 |
| Atorvastatin related compound Bc | 0.94 | 1.0 | 0.3 |
| Atorvastatin | 1.0 | — | — |
| Atorvastatin related compound Cd | 1.1 | 1.0 | 0.3 |
| Atorvastatin 3-deoxyhept-2-enoic acide | 1.45 | 1.0 | 0.10 |
| Atorvastatin related compound Hf | 1.90 | 1.0 | 0.15 |
| Atorvastatin epoxy tetrahydrofuran analogg | 2.00 | 0.71 | 0.15 |
| Atorvastatin ethyl esterh | 2.08 | 1.0 | 0.15 |
| Atorvastatin related compound Di | 2.18 | 1.3 | 0.15 |
| Atorvastatin related compound Ij | 2.75 | 1.0 | 0.15 |
| Any other individual impurity | — | 1.0 | 0.10 |
| Total impuritiesk | — | — | 1.0 |
a (3R,5R)-7-{(3R,5R)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanamido}-3,5-dihydroxyheptanoic acid.
b Desfluoro impurity.
c 3S,5R Isomer.
d Difluoro impurity.
e (S,E)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-5-hydroxyhept-2-enoic acid.
f Lactone impurity.
g 4-(4-Fluorophenyl)-2,4-dihydroxy-2-isopropyl-N,5-diphenyl-3,6-dioxabicyclo[3.1.0]hexane-1-carboxamide.
h (3R,5R)-Ethyl 7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate.
i Epoxide impurity.
j Acetonide impurity.
k This total does not include atorvastatin related compound E, as determined in the Enantiomeric Purity test.
6 ENANTIOMERIC PURITY
Mobile phase: Hexane, dehydrated alcohol, and trifluoroacetic acid (940:60:1)
System suitability stock solution: 5 mg/mL of USP Atorvastatin Calcium RS and 37.5 µg/mL of USP Atorvastatin Related Compound E RS in methanol.
[Note—Atorvastatin related compound E is the 3S,5S enantiomer of atorvastatin.]
System suitability solution: Transfer 2.0 mL of the System suitability stock solution to a 10-mL volumetric flask, add 2.0 mL of dehydrated alcohol, and dilute with hexane to volume.
Sample solution: Transfer 10 mg of Atorvastatin Calcium to a 10-mL volumetric flask, dissolve in 2.0 mL of methanol, add 2.0 mL of dehydrated alcohol, and dilute with hexane to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 244 nm
Column: 4.6-mm × 25-cm; packing L51
Flow rate: 1.0 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[Note—The elution order of the peaks is atorvastatin related compound E followed by atorvastatin.]
Resolution: NLT 2.0 between the peaks for atorvastatin related compound E and atorvastatin
Analysis
Sample: Sample solution
Calculate the percentage of atorvastatin related compound E in the portion of Atorvastatin Calcium taken:
Result = (ru/rT) × 100
ru = peak response for atorvastatin related compound E
rT = sum of the peak responses for atorvastatin related compound E and atorvastatin
Acceptance criteria: NMT 0.3% of atorvastatin related compound E
7 SPECIFIC TESTS
Water Determination, Method Ia 〈921〉: 3.5%–5.5% for the trihydrate form. If labeled as amorphous or as semicrystalline, NMT 6.0%. If labeled as a propylene glycol solvate, NMT 1.0%.
8 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve the trihydrate form in well-closed containers, and store at room temperature. If labeled as amorphous or semicrystalline or as a propylene glycol solvate, store as per labeling instructions. Possible packaging and storage conditions could include the following: Preserve in well-closed containers protected from light and moisture, or in tight containers; store at room temperature, at controlled room temperature, or at 2°–8°; store under nitrogen atmosphere or packed with an oxygen absorber; and store under nitrogen atmosphere, packed with silica gel and an oxygen absorber.
Labeling: Where it is an amorphous form, the label so indicates. Where it is a semicrystalline form, the label so indicates. Where it is a propylene glycol solvate form, the label so indicates. If a test for Organic Impurities other than Procedure 1 is used, the labeling states the test with which the article complies. Label it to indicate the name and quantity of any added antioxidant.
Change to read:
USP Reference Standards 〈11〉
USP Atorvastatin Calcium RS
USP Atorvastatin Related Compound A RS
Calcium (3R,5R)-7-[2-isopropyl-4,5-diphenyl-3-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate (1:2);
Also known as Desfluoro impurity, or (3R,5R)-7-[3-(phenylcarbamoyl)-2-isopropyl-4,5-diphenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid, calcium salt.
C₆₆H₇₀CaN₄O₁₀ 1119.38
USP Atorvastatin Related Compound B RS
Calcium (3S,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate (1:2);
Also known as 3S,5R Isomer, or (3S,5R)-7-[3-(phenylcarbamoyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid, calcium salt.
C₆₆H₆₈CaF₂N₄O₁₀ 1155.34
USP Atorvastatin Related Compound C RS
Calcium (3R,5R)-7-[2,3-Bis(4-fluorophenyl)-5-isopropyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate (1:2);
Also known as Difluoro impurity, or (3R,5R)-7-[3-(phenylcarbamoyl)-4,5-bis(4-fluorophenyl)-2-isopropyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid, calcium salt.
C₆₆H₆₆CaF₄N₄O₁₀ 1191.34
USP Atorvastatin Related Compound D RS
3-(4-Fluorobenzoyl)-2-isobutyryl-N,3-diphenyloxirane-2-carboxamide;
Also known as Epoxide impurity, or 3-(4-fluorobenzoyl)-2-isobutyryl-3-phenyl-oxirane-2-carboxylic acid phenylamide.
C₂₆H₂₂FNO₃ 431.46
USP Atorvastatin Related Compound E RS
Calcium (3S,5S)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate (1:2);
Also known as 3S,5S Enantiomer, or (3S,5S)-7-[3-(phenylcarbamoyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid, calcium salt.
C₆₆H₆₈CaF₂N₄O₁₀ 1155.36
USP Atorvastatin Related Compound H RS
Also known as Lactone impurity;
(ERR 1-Jun-2023)
5-(4-Fluorophenyl)-1-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-2-isopropyl-N,4-diphenyl-1H-pyrrole-3-carboxamide.
C₃₃H₃₅F N₂O₄ 540.64
USP Atorvastatin Related Compound I RS
Also known as Acetonide impurity;
(ERR 1-Jun-2023)
tert-Butyl 2-((4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxan-4-yl)acetate.
C₄₀H₄₇FN₂O₅ 654.82


