Aspirin, Alumina, and Magnesium Oxide Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Aspirin, Alumina, and Magnesium Oxide Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of aspirin (C9H8O4), the equivalent of NLT 90.0% and NMT 110.0% of the labeled amount of aluminum hydroxide [Al(OH)3], and NLT 90.0% and NMT 110.0% of the labeled amount of magnesium oxide (MgO).
2 IDENTIFICATION
SAMPLE: The Sample is prepared as follows. To a 0.7-g portion of finely powdered Tablets, add 20 mL of 3 N hydrochloric acid and 5 drops of methyl red TS, heat to boiling, and add 6 N ammonium hydroxide until the color of the solution changes to deep yellow. Continue boiling for 2 min, and filter. The filtrate is used in Identification test B, and the precipitate is used in Identification test C.
A. The retention time of the aspirin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. IDENTIFICATION TESTS GENERAL, Magnesium(191).
Sample solution: Sample filtrate
Acceptance criteria: Meet the requirements
C. IDENTIFICATION TESTS-GENERAL, Aluminum (191)
Sample solution: Wash the Sample precipitate with a hot solution of 20 mg/mL of ammonium chloride, and dissolve the precipitate in hydrochloric acid.
Acceptance criteria: Meet the requirements
D. PROCEDURE
Analysis: Where the Tablets are composed of two layers, scrape a small amount of each layer into separate test tubes. Add 2 mL of water and 2 drops of methyl red TS to each tube, and shake for 15 s.
Acceptance criteria: The solution from the aspirin-containing layer is red, and the solution from the buffer-containing layer is yellow.
3 ASSAY
3.1 ASPIRIN
Mobile phase: Methanol, phosphoric acid, and water (30:3:70)
Diluent: Dehydrated alcohol and hydrochloric acid (2000:20)
Aspirin standard stock solution: 5 mg/mL of USP Aspirin RS in Diluent prepared by blending at high speed for 1.5 min
Aspirin standard solution: 0.25 mg/mL of USP Aspirin RS prepared immediately from the Aspirin standard stock solution in dehydrated alcohol. Use these solutions within 1 h.
Salicylic acid standard stock solution: 5 mg/mL of USP Salicylic Acid RS in dehydrated alcohol. Transfer 3.0 mL of this solution to a 100-mL
volumetric flask, and dilute with Diluent to volume.
Salicylic acid standard solution: 7.5 µg/mL of USP Salicylic Acid RS from the Salicylic acid standard stock solution in dehydrated alcohol
System suitability solution: Transfer 5.0 mL of the Aspirin standard stock solution to a 100-mL volumetric flask, add 5.0 mL of the Salicylic
acid standard stock solution, and dilute with dehydrated alcohol to volume.
Sample solution: Transfer a counted number of Tablets, equivalent to 2500 mg of aspirin, to a 120-mL blender jar containing 100.0 mL of Diluent, and blend at high speed for 1.5 min. Immediately filter a portion of the mixture thus obtained, and transfer 1.0 mL of the filtrate to a 100-mL volumetric flask. Immediately dilute with dehydrated alcohol to volume. Promptly inject this Sample solution into the chromatograph as directed for Analysis.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.6-mm x 3-cm; 5-µm packing L7
Flow rate: 3.5 mL/min
Injection volume: 10 µL.
System suitability
Samples: Aspirin standard solution, Salicylic acid standard solution, and System suitability solution
[NOTE-The relative retention times for aspirin and salicylic acid are 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution: NLT 2.0 between the aspirin peak and the salicylic acid peak, System suitability solution
Tailing factor: NMT 2.0 for the aspirin and salicylic acid peaks, Aspirin standard solution and Salicylic acid standard solution
Relative standard deviation: NMT 2.0% for the aspirin and salicylic acid peaks, Aspirin standard solution and Salicylic acid standard solution
Analysis
Samples: Aspirin standard solution, Salicylic acid standard solution, and Sample solution
Calculate the percentage of aspirin (C9H8O4) in each Tablet taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of aspirin from the Sample solution
rs = peak response of aspirin from the Standard solution
Cs = concentration of USP Aspirin RS in the Aspirin standard solution (mg/mL)
Cu = nominal concentration of aspirin in the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
3.2 ALUMINUM HYDROXIDE
Edetate disodium titrant: Dissolve 18.6 g of edetate disodium in water to make 1000 mL, and standardize the solution as follows. Weigh 2 g
of aluminum wire, transfer to a 1000-mL volumetric flask, and add 50 mL of a mixture of hydrochloric acid and water (1:1). Swirl the flask to ensure contact of the aluminum and the acid, and allow the reaction to proceed until all of the aluminum has dissolved. Dilute with water to volume. Pipet 10 mL of this solution into a 250-ml beaker; add, in the order named and with continuous stirring, 25.0 mL of edetate disodium titrant and 20 mL of acetic acid-ammonium acetate buffer TS; and boil gently for 5 min. Cool, and add 50 mL of alcohol and 2 mL of dithizone TS. Titrate with 0.05 M zinc sulfate VS to a bright rose-pink color. Perform a blank determination, substituting 10 mL of water for the aluminum solution, and make any necessary correction.
Calculate the molarity of the solution taken:
Result = (W/Ar ) × V
W = weight of aluminum in the portion of the solution taken (mg)
Ar = atomic weight of aluminum, 26.98
V = volume of Edetate disodium titrant consumed (mL)
Sample solution: To a portion of the powdered Tablets (NLT 20) equivalent to 600 mg of aluminum hydroxide, add 20 mL of water, stir, and slowly add 30 mL of 3 N hydrochloric acid. Heat gently, if necessary, to aid solution, cool, and transfer to a 200-mL volumetric flask. Wash the beaker with water, adding the washings to the flask, and add water to volume.
Analysis: To 20 mL of the Sample solution in a 250 ml-beaker, add 20 mL of water, then add, in the order named and with continuous stirring, 25.0 mL of Edetate disodium titrant and 20 mL of acetic acid-ammonium acetate buffer TS, and heat the solution near the boiling temperature for 5 min. Cool, and add 50 mL of alcohol and 2 mL of dithizone TS. Titrate with 0.05 M zinc sulfate VS until the color changes from green-violet to rose-pink. Perform a blank determination, substituting 10 mL of water for the Sample solution, and make any necessary correction. Each mL of 0.05 M Edetate disodium titrant is equivalent to 3.900 mg of aluminum hydroxide [Al(OH)3].
Acceptance criteria: 90.0%-110.0%
3.3 MAGNESIUM OXIDE
Sample solution: Prepare as directed in the Assay for Aluminum Hydroxide.
Eriochrome black indicator solution: Dissolve 200 mg of eriochrome black T in a mixture of 15 mL of triethanolamine and 5 mL of dehydrated alcohol.
Titrant: 0.05 M edetate disodium VS
Analysis: To a volume of the Sample solution equivalent to 40 mg of magnesium oxide in a 400 mL beaker, add, while mixing, 20 mL of triethanolamine and 200 mL of water. Cool the solution for 10 min, while stirring, by immersion in an ice bath. Remove the beaker from the ice bath, and add 15 mL of ammonia-ammonium chloride buffer TS and 2 drops of Eriochrome black indicator solution. Titrate with Titrant to a blue endpoint, allowing 60 s between drops of Titrant as the endpoint is approached (after first color change is observed). The titration should be completed within 10 min after the addition of the buffer and indicator. If any precipitate is observed prior to titration, the solution should be discarded, and a new solution prepared. Perform a blank determination, substituting an equivalent volume of water for the volume of Sample solution used, and make any necessary correction. Each mL of Titrant consumed is equivalent to 2.015 mg of magnesium oxide (MgO).
Acceptance criteria: 90.0%-110.0%
4 PERFORMANCE TESTS
DISSOLUTION (711)
Medium: 0.05 M acetate buffer, prepared by mixing 2.99 g of sodium acetate (trihydrate) and 1.66 mL of glacial acetic acid with water to obtain 1000 mL of solution having a pH of 4.50 ± 0.05; 900 mL
Apparatus 1 (10-mesh screen): 100 rpm
Time: 45 min
Determine the amount of aspirin (C₂H₂O) dissolved, employing the following method.
Alkaline detergent solution: 30% solution of polyoxyethylene (23) lauryl ether and 1 N sodium hydroxide (0.5:1000)
pH 4.3 buffer detergent: 12.9 g/L of citric acid monohydrate and 20.6 g/L of dibasic sodium phosphate heptahydrate in water. Add 0.5 mL of
a 30% solution of polyoxyethylene (23) lauryl ether.
Standard solution: 0.45 mg/mL of USP Aspirin RS in Medium
Sample solution: Filtered portion of sample
Analysis: Use an automatic analyzer consisting of (1) a liquid sampler, (2) a proportioning pump, (3) a suitable fluorometer equipped with a 0.4-cm flow cell and suitable recording devices, and (4) a manifold consisting of the components illustrated in Figure 1.
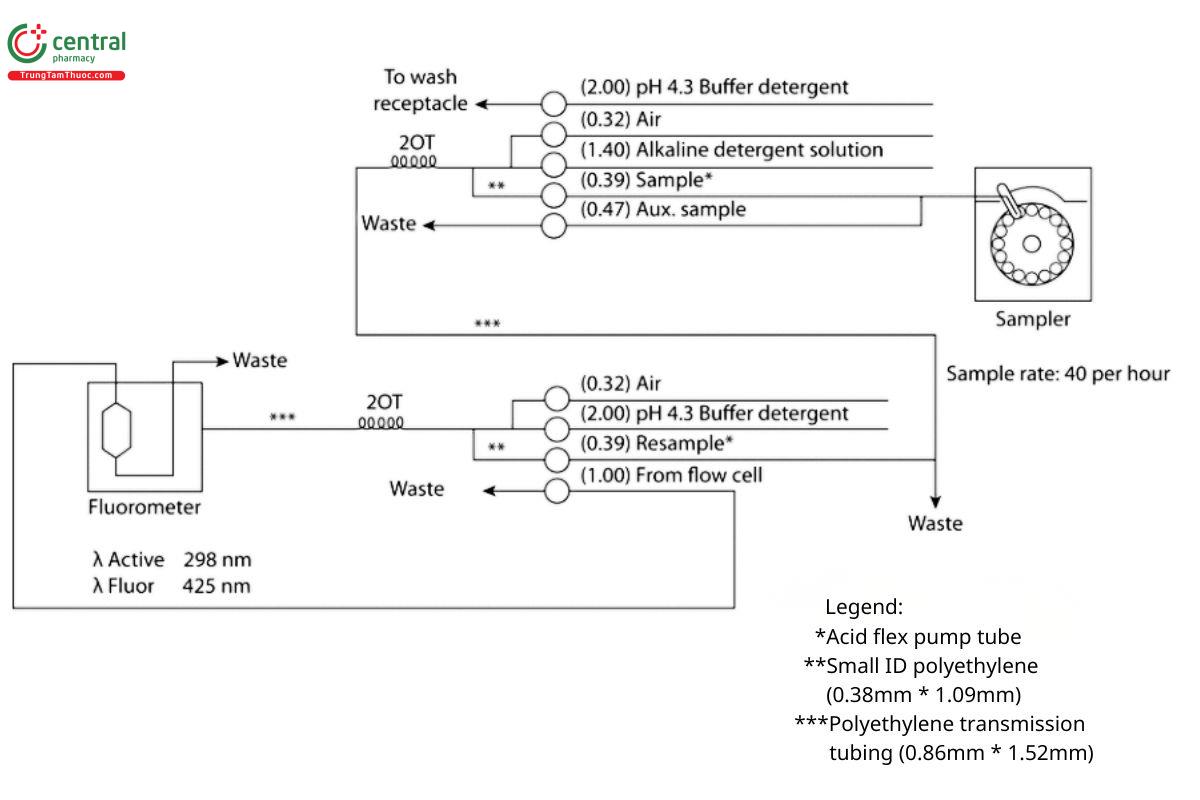
Result = Cs x (V/L) × (Fu/Fs) × 100
Cs = concentration of USP Aspirin RS in the Standard solution (mg/mL)
V = volume of medium, 900 mL
L = label claim (mg/Tablet)
Fu = fluorescence values of the solution under test
Fs = fluorescence values of the Standard solution
Tolerances: NLT 75% (Q) of the labeled amount of aspirin (C9H8O4) is dissolved.
UNIFORMITY OF DOSAGE UNITS (905): Meet the requirements for Weight Variation with respect to aluminum hydroxide and to magnesium oxide, and for Content Uniformity with respect to aspirin
5 IMPURITIES
ORGANIC IMPURITIES PROCEDURE: LIMIT OF FREE SALICYLIC ACID
[NOTE-The results from the Assay for Aspirin may be used for this test when calculated as described in the Analysis section of this test.]
Mobile phase: Methanol, phosphoric acid, and water (30:3:70)
Diluent: Dehydrated alcohol and hydrochloric acid (2000:20)
Aspirin standard stock solution: 5 mg/mL of USP Aspirin RS in Diluent prepared by blending at high speed for 1.5 min
Aspirin standard solution: 0.25 mg/mL of USP Aspirin RS prepared immediately from Aspirin standard stock solution in dehydrated alcohol.
Use these solutions within 1 h.
Salicylic acid standard stock solution: 5 mg/mL of USP Salicylic Acid RS in dehydrated alcohol. Transfer 3.0 mL of this solution to a 100-mL
volumetric flask, and dilute with Diluent to volume.
Salicylic acid standard solution: 7.5 µg/mL of USP Salicylic Acid RS from Salicylic acid standard stock solution in dehydrated alcohol
System suitability solution: Transfer 5.0 mL of the Aspirin standard stock solution to a 100-mL volumetric flask, add 5.0 mL of the Salicylic acid standard stock solution, and dilute with dehydrated alcohol to volume.
Sample solution: Transfer a counted number of Tablets, equivalent to 2500 mg of aspirin, to a 120-mL blender jar containing 100.0 mL of Diluent, and blend at high speed for 1.5 min. Immediately filter a portion of the mixture thus obtained, and transfer 1.0 mL of the filtrate to a 100-mL volumetric flask. Immediately dilute with dehydrated alcohol to volume. Promptly inject this Sample solution into the chromatograph as directed for Analysis.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.6-mm x 3-cm; 5-µm packing L7
Flow rate:
3.5 mL/min.
Injection volume: 10 µL
System suitability
Samples: Aspirin standard solution, Salicylic acid standard solution, and System suitability solution [NOTE-The relative retention times for aspirin and salicylic acid are 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution: NLT 2.0 between the aspirin and salicylic acid peaks, System suitablity solution
Tailing factor: NMT 2.0 for the aspirin and salicylic acid peaks, Aspirin standard solution and Salicylic acid standard solution
Relative standard deviation: NMT 2.0% for aspirin and salicylic acid peaks, Aspirin standard solution and Salicylic acid standard solution
Analysis
Samples: Aspirin standard solution, Salicylic acid standard solution, and Sample solution
Calculate the percentage of free salicylic acid in the Tablets taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of salicylic acid from the Sample solution
rs = peak response from the Salicylic acid standard solution
Cs = concentration of USP Salicylic Acid RS in the Salicylic acid standard solution (µg/mL)
Cu = nominal concentration of aspirin in the Sample solution (mg/mL)
Acceptance criteria: NMT 3.0%
6 SPECIFIC TESTS
Acid-Neutralizing Capacity 〈301〉: NLT 1.9 mEq of acid is consumed for each 325 mg of aspirin in the Tablets.
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Aspirin RS
USP Salicylic Acid RS


