Ascorbic Acid
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
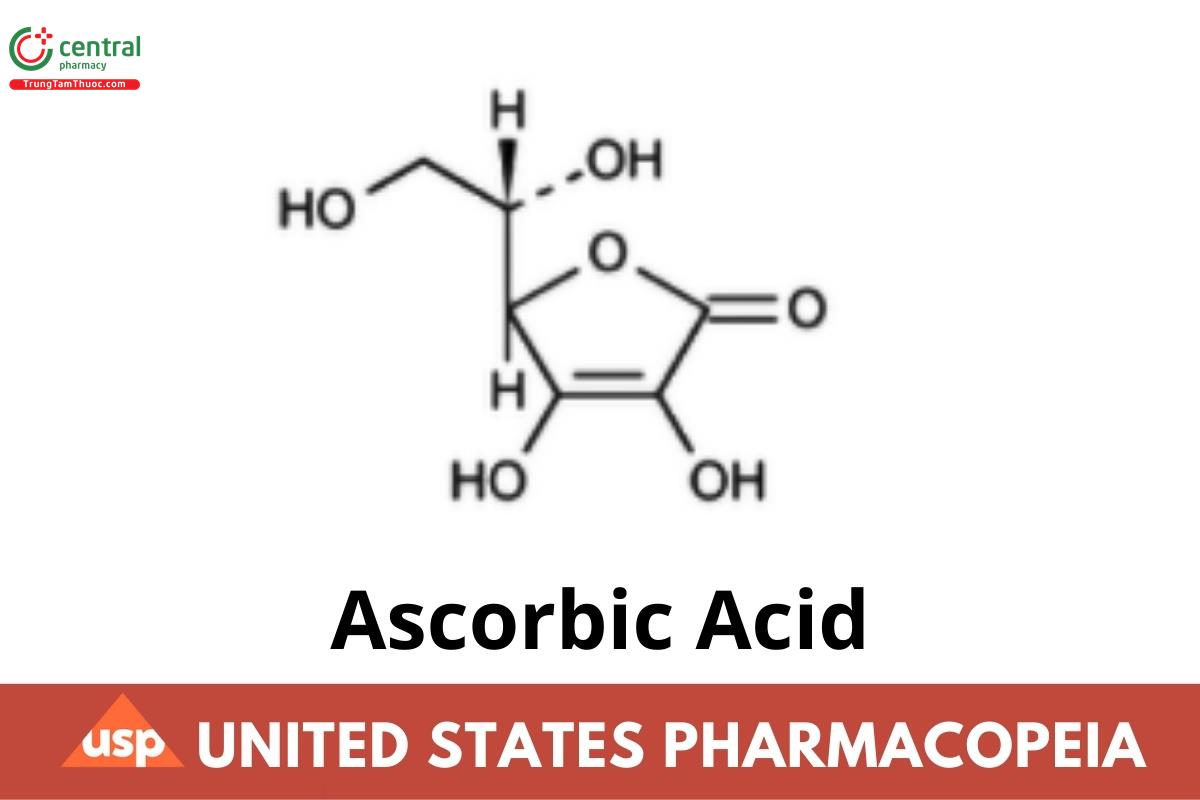
C6H8O6 176.12
L-Ascorbic acid CAS RN: 50-81-7.
1 DEFINITION
Ascorbic Acid contains NLT 99.0% and NMT 100.5% of C6H8O6.
2 IDENTIFICATION
Change to read:
A. ASPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. A 20-mg/mL solution reduces alkaline cupric tartrate TS slowly at room temperature but more readily upon heating.
3 ASSAY
3.1 PROCEDURE
Sample: 400 mg of Ascorbic Acid
Titrimetric system (See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.1 N iodine VS
Endpoint detection: Visual
Blank: 100 mL of water and 25 mL of 2N sulfuric acid. Add 3 mL of starch TS.
Analysis: Dissolve the Sample in a mixture of 100 mL of water and 25 mL of 2 N sulfuric acid. Add 3 mL of starch TS, and titrate immediately with Titrant until a persistent violet-blue color is obtained.
Calculate the percentage of ascorbic acid (C6H8O6) in the portion of Ascorbic Acid taken:
Result = [(V - B) × N × F × 100]/W
V = sample titrant volume (mL)
B = blank titrant volume (mL)
N = titrant normality (mEq/mL)
F = equivalency factor, 88.06 mg/mEq
W = weight of Sample (mg)
Acceptance criteria: 99.0%-100.5%
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
5 SPECIFIC TESTS
OPTICAL ROTATION, Specific Rotation(781S).
Sample solution: 100 mg/mL in carbon dioxide-free water. Perform the test immediately after preparation of the Sample solution.
Acceptance criteria: +20.5° to +21.5°
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
USP REFERENCE STANDARDS (11).
USP Ascorbic Acid RS


