Apomorphine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
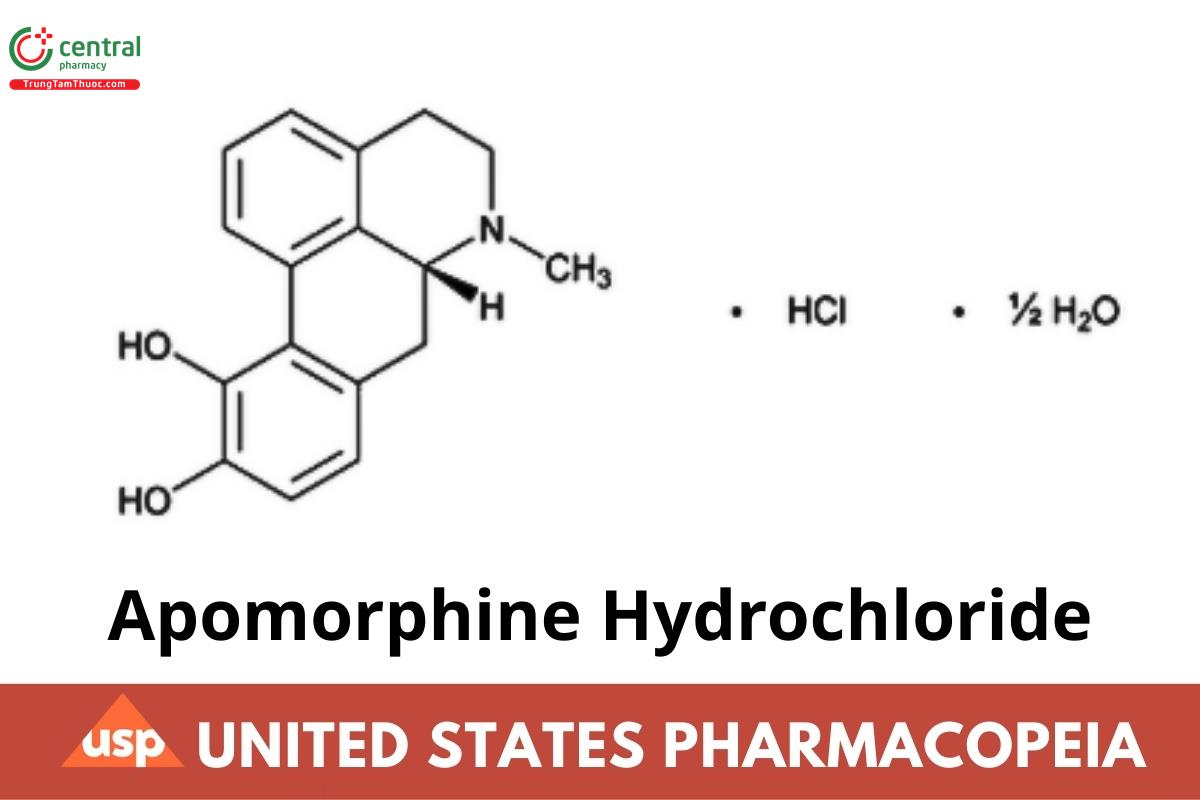
C17H17NO2 . HCI 1/2H2O 312.79
C17H17NO2 . HCI 303.79
4H-Dibenzo[de,g]quinoline-10,11-diol, 5,6,6a, 7-tetrahydro-6-methyl-, hydrochloride, hemihydrate, (R)-;
6aß-Aporphine-10,11-diol hydrochloride hemihydrate CAS RN®: 41372-20-7; UNII: F39049Y068.
Anhydrous CAS RN®: 314-19-2; UNII: 9K13MD7A0D.
1 DEFINITION
Apomorphine Hydrochloride contains NLT 98.5% and NMT 101.5% of apomorphine hydrochloride (C17H17NO2 . HCI), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
2.2 B. IDENTIFICATION TESTS-GENERAL, Chloride (191)
Sample solution: 10 mg/mL of Apomorphine Hydrochloride in carbon dioxide-free water
Analysis: To 2 mL of the Sample solution add 0.1 mL of nitric acid. Mix, filter, and use the filtrate.
Acceptance criteria: Meets the requirements
3 ASSAY
3.1 PROCEDURE
Sample solution: Dissolve 250 mg of Apomorphine Hydrochloride in a mixture of 5.0 mL of 0.01 N hydrochloric acid and 50 mL of alcohol.
Analysis: Titrate the Sample solution with 0.1 N sodium hydroxide VS. Read the volume added between the first two points of inflexion. Each mL of 0.1 N sodium hydroxide is equivalent to 30.38 mg of apomorphine hydrochloride (C17H17NO2 . HCI).
Acceptance criteria: 98.5%-101.5% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281): NMT 0.1%
4.2 ORGANIC IMPURITIES
Diluent: Glacial acetic acid and water (1:99)
Solution A: 1.1-g/L solution of sodium octanesulfonate, adjusted with diluted phosphoric acid (1:1) to a pH of 2.2
Solution B: Acetonitrile
Mobile phase: See Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 85 | 15 |
| 2 | 85 | 15 |
| 32 | 68 | 32 |
| 37 | 68 | 32 |
Return to original conditions and re-equilibrate the system.
System suitability solution: 0.25 mg/mL each of USP Apomorphine Hydrochloride RS and boldine in Diluent. [NOTE-Boldine is 2,9-dihydroxy-1,10-dimethoxyaporphine.]
Standard solution: 2.5 µg/mL of USP Apomorphine Hydrochloride RS in Diluent
Sensitivity solution: 0.14 µg/mL of USP Apomorphine Hydrochloride RS in Diluent from the Standard solution. [NOTE-The peak response of this solution is equivalent to that of a solution containing 1.25 µg/mL of morphine hydrochloride, taking into account the relative response factor of this impurity (see Table 2).]
Sample solution: 2.5 mg/mL of Apomorphine Hydrochloride in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 15-cm; 5-µm end-capped packing L1
Column temperature: 35°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Sensitivity solution
[NOTE-The typical relative retention times for boldine and apomorphine are about 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.5 between boldine and apomorphine, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Apomorphine Hydrochloride taken: Result = (rU/rS) × (CS/CU) × (1/F) x 100
rU = peak response of each impurity from the Sample solution
rS = peak response of apomorphine from the Standard solution
CS = concentration of USP Apomorphine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Apomorphine Hydrochloride in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. Disregard any peak below 0.05%.
Table 2
| Name | Relative Retention Time | Response Factor Relative | Acceptance Criteria, NMT (%) |
| Morphine | 0.4 | 0.11 | 0.15 |
| Apomorphine | 1.0 | - | - |
| Any other individual impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 0.5 |
5 SPECIFIC TESTS
5.1 OPTICAL ROTATION, Specific Rotation (781S).
Sample solution: 10 mg/mL in Diluent
Diluent: 2.06-g/L solution of hydrochloric acid in water
Acceptance criteria: 48° to 52°, determined at 20°
5.2 LOSS ON DRYING (731).
Analysis: Dry a sample at 105° for 2 h.
Acceptance criteria: 2.0%-4.2%
5.3 COLOR OF SOLUTION
Sample solution: Place 100 mg of Apomorphine Hydrochloride in a suitable test tube, add 10 mL of cold, oxygen-free water, and agitate gently until dissolved.
Standard solution: Dissolve 5 mg of Apomorphine Hydrochloride in 100.0 mL of water. Transfer 1.0 mL of this solution to a test tube of the same size as that used for the Sample solution. Dilute with 6 mL of water, add 1 mL of a 50-mg/mL sodium bicarbonate solution, and then add 0.50 mL of iodine TS. Allow to stand for 30 s, add 0.60 mL of a 25-mg/mL sodium thiosulfate solution, and dilute with water to 10 mL.
Acceptance criteria: The color of the Sample solution, observed promptly after the Apomorphine Hydrochloride has dissolved, is not more intense than that of a color of the Standard solution.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
USP REFERENCE STANDARDS (11)
USP Apomorphine Hydrochloride RS


