Ampicillin Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Ampicillin Tablets
1 DEFINITION
Ampicillin Tablets contain an amount of Ampicillin (anhydrous form or trihydrate form) equivalent to NLT 90.0% and NMT 120.0% of the labeled amount of ampicillin (C₁₆H₁₉N₃O₄S).
2 IDENTIFICATION
A. Thin-Layer Chromatography
Diluent: Acetone and 0.1 N hydrochloric acid (4:1)
Standard solution: 5 mg/mL of USP Ampicillin RS in Diluent
Sample solution: 5 mg/mL of ampicillin from powdered Tablets in Diluent
Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 2 µL
Developing solvent system: Acetone, toluene, glacial acetic acid, and water (650:100:25:100)
Spray reagent: 3 mg/mL of ninhydrin in alcohol
Analysis
Samples: Standard solution and Sample solution
Apply the Standard solution and the Sample solution to the plate, and develop the chromatogram using the Developing solvent system. When the solvent front has moved about three-fourths of the length of the plate, remove the plate from the chamber, mark the solvent front, and allow to air-dry. Locate the spots on the plate by spraying lightly with the Spray reagent, and dry at 90° for 15 min.
Acceptance criteria: The R value of the principal spot of the Sample solution corresponds to that of the Standard solution.
3 ASSAY
Procedure
Standard solution: Prepare as directed for Standard Preparation in Iodometric Assay—Antibiotics 〈425〉, using USP Ampicillin RS.
Sample solution: Place NLT 5 Tablets in a high-speed glass blender jar containing an accurately measured volume of water, and blend for 4 ± 1 min. Dilute a suitable aliquot with water to obtain a concentration of 1.25 mg/mL of ampicillin.
Analysis
Samples: Standard solution and Sample solution
Proceed as directed for Procedure in Iodometric Assay—Antibiotics 〈425〉.
Calculate the percentage of the labeled amount of ampicillin (C₁₆H₁₉N₃O₄S) in the portion of Tablets taken:
Result = (B − I) × (F1 /2) × (1/Cu) × F2 × 100
B = volume of 0.01 N sodium thiosulfate consumed in the Blank Determination (mL)
I = volume of 0.01 N sodium thiosulfate consumed in the Inactivation and Titration of the Sample solution (mL)
F1 = factor as calculated in Iodometric Assay—Antibiotics 〈425〉
Cu = nominal concentration of ampicillin in the Sample solution (mg/mL)
F2 = conversion factor, 0.001 mg/µg
Acceptance criteria: 90.0%–120.0%
4 PERFORMANCE TESTS
Dissolution, Procedure for a Pooled Sample 〈711〉
Medium: Water; 900 mL
Apparatus 1: 100 rpm
Time: 45 min
Standard solution: L/900 mg/mL of USP Ampicillin RS in water, where L is the labeled amount of ampicillin in mg/Tablet
Sample solution: Use a filtered portion of the solution under test.
Solution A: 1 in 1000 solution of polyoxyethylene (23) lauryl ether in water
Solution B: Dissolve 20 g of hydroxylamine hydrochloride in 5 mL of Solution A, and add water to make 1000 mL.
Buffer: 26 mg/mL of sodium hydroxide and 3.1 mg/mL of sodium acetate in water
Ferric nitrate solution: Suspend 233 g of ferric nitrate in about 600 mL of water, add 2.8 mL of sulfuric acid, stir until the ferric nitrate is dissolved, add 1 mL of polyoxyethylene (23) lauryl ether, dilute with water to 1000 mL, and mix.
Apparatus: Automatic analyzer consisting of (1) a liquid sampler, (2) a proportioning pump, (3) suitable spectrophotometers equipped with matched flow cells and analysis capability at 480 nm, (4) a means of recording spectrophotometric readings, and/or computer for data retrieval and calculation, and (5) a manifold consisting of the components illustrated in Figure 1.
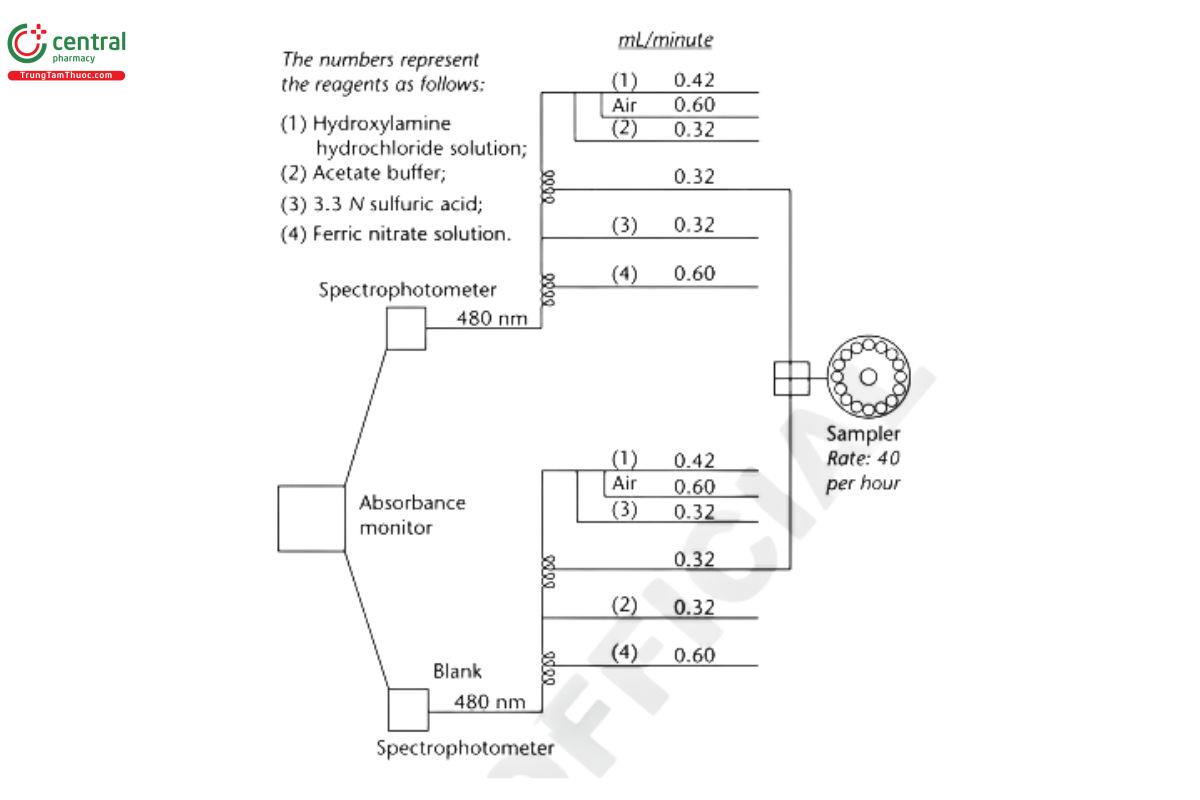
Analysis
Samples: Standard solution and Sample solution
With the sample line pumping water, the other lines pumping their respective reagents, and the spectrophotometer set at 480 nm, standardize the system until a steady absorbance baseline has been established. Transfer portions of the Standard solution and the Sample solution to sampler cups, and place in the sampler. Start the sampler, and conduct determinations of the Standard solution and the Sample solution typically at the rate of 40/h using a ratio of about 2:1 for sample and wash time.
Calculate the percentage of the labeled amount of ampicillin (C₁₆H₁₉N₃O₄S) dissolved:
Result = (Aᵤ/Aₛ) × Cₛ × V × P × F × (1/L) × 100
Aᵤ = response of the Sample solution
Aₛ = response of the Standard solution
Cₛ = concentration of USP Ampicillin RS in the Standard solution (mg/mL)
V = volume of medium, 900 mL
P = potency of ampicillin in USP Ampicillin RS (µg/mg)
F = conversion factor, 0.001 mg/µg
L = label claim (mg/Tablet)
Tolerances: NLT 75% (Q) of the labeled amount of ampicillin (C₁₆H₁₉N₃O₄S) is dissolved.
Uniformity of Dosage Units 〈905〉: Meet the requirements
5 SPECIFIC TESTS
Water Determination, Method I 〈921〉
| Type of Tablets | Form of Ampicillin | Limit (%) |
| Nonchewable | Anhydrous | NMT 4.0 |
| Nonchewable | Trihydrate | 9.5–12.0 |
| Chewable | Anhydrous | NMT 3.0 |
| Chewable | Trihydrate | NMT 5.0 |
| Tablets labeled for veterinary use only | Trihydrate | NMT 13.0 |
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
Labeling: Label the Tablets to indicate whether the ampicillin therein is in the anhydrous form or is the trihydrate. Label chewable Tablets to indicate that they are to be chewed before swallowing. Tablets intended for veterinary use only are so labeled.
USP Reference Standards 〈11〉
USP Ampicillin RS


