Ammonium Glycyrrhizate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
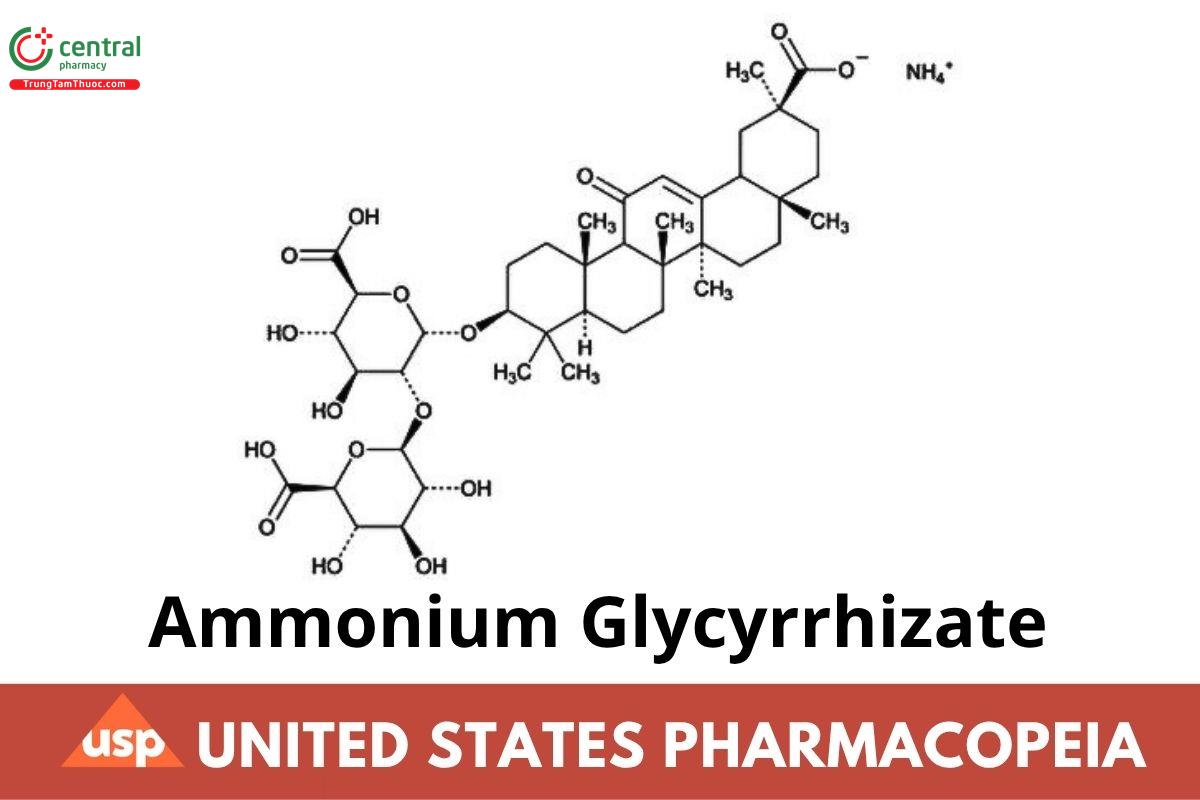
C42H62O16 . NH3 839.97 (ERR 1-Jul-2022)
Monoammonium glycyrrhizinate;
Glycyrrhizic acid ammonium salt;
α-D-Glucopyranosiduronic acid, (3β,20β)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-β-D-glucopyranuronosyl-, ammonium salt (1:1);
α-D-Glucopyranosiduronic acid, (3β,20β)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-β-D-glucopyranuronosyl-, monoammonium salt CAS
RN: 53956-04-0.
1 DEFINITION
Ammonium Glycyrrhizate is a mixture of ammonium 18α- and 18β-glycyrrhizate (ammonium salt of (20β)-3β-[[2-0-(β-D-glucopyranosyluronic acid)-α-D-glucopyranosyluronic acid oxy]-11-oxoolean-12-en-29-oic acid), and the 18β-isomer is the main component. It contains NLT 78.0% and NMT 102.0% of ammonium 18α- and 18β-glycyrrhizate, on the anhydrous basis.
2 IDENTIFICATION
A. The retention times of the peaks of 18α- and 18β-glycyrrhizic acid from the Sample solution correspond to those from the System suitability solution, as obtained in the Content of Ammonium 18α- and 18β-Glycyrrhizate. [NOTE-The peak of 18α-glycyrrhizic acid could be absent in the Sample solution.]
B. IDENTIFICATION TESTS-GENERAL, Ammonium (191)
Acceptance criteria: Meets the requirements
3 ASSAY
Change to read:
3.1 CONTENT OF AMMONIUM 18α AND 18β-GLYCYRRHIZATE
3.1.1 Mobile phase
Acetonitrile, glacial acetic acid, and water (38:1:61)
3.1.2 Standard solution
0.5 mg/mL of USP Glycyrrhizic Acid RS in Mobile phase
3.1.3 System suitability solution
0.5 mg/mL of USP Ammonium Glycyrrhizate RS in Mobile phase
3.1.4 Sample solution
0.5 mg/mL of Ammonium Glycyrrhizate in Mobile phase
3.1.5 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm x 30-cm analytical column; 5-10 µm packing L1
Flow rate: 2.0 mL/min
Injection volume: 10 µL
3.1.6 System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times of 18β-glycyrrhizic acid and 18α-glycyrrhizic acid are about 1.0 and 1.2, respectively, System suitability solution.]
Suitability requirements
- Resolution: NLT 2.0 between the peaks due to 18β-glycyrrhizic acid and 18α-glycyrrhizic acid, System suitability solution
- Relative standard deviation: NMT 2.0%, Standard solution
3.1.7 Analysis
3.1.7.1 Samples
Standard solution, System suitability solution, and Sample solution
Determine the peak areas for each isomer (18α-glycyrrhizic acid or 18β-glycyrrhizic acid).
Calculate the percentage of ammonium 18α-glycyrrhizate (or ammonium 18β-glycyrrhizate) in the portion of Ammonium Glycyrrhizate
taken:
Result = (ru/rs) × (Cs/Cu) × (MW(Salt)/MW(Acid)) × 100
ru = peak area of the 18α-glycyrrhizic acid (or 18β-glycyrrhizic acid) in the Sample solution
rs = peak area of the 18β-glycyrrhizic acid in the Standard solution
Cs = concentration of the USP Glycyrrhizic Acid RS in the Standard solution (mg/mL)
Cu = concentration of the Sample solution (mg/mL)
MW(Salt) = molecular weight of ammonium glycyrrhizate, 839.97 (ERR 1-Jul-2022) g/mol
MW(Acid) = molecular weight of glycyrrhizic acid, 822.94 (ERR 1-Jul-2022) g/mol
3.1.7.2 Acceptance criteria
The total percentage of ammonium 18α-glycyrrhizate and ammonium 18β-glycyrrhizate is 78.0%-102.0%, and the percentage of ammonium 18α-glycyrrhizate is NMT 13.0%, on the anhydrous basis.
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
Sample: 1.0 g
Acceptance criteria: NMT 0.5%
4.2 LIMIT OF ORGANIC IMPURITIES
Mobile phase, System suitability solution, and Chromatographic system: Proceed as directed in Content of Ammonium 18α- and 188-
Glycyrrhizate.
Sample solution: 1.0 mg/mL of Ammonium Glycyrrhizate in the Mobile phase
Reference solution A: 0.05 mg/mL of Ammonium Glycyrrhizate in the Mobile phase, prepared from the Sample solution
Reference solution B: 0.057 mg/mL of Ammonium Glycyrrhizate in the Mobile phase, prepared from the Sample solution
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for 24-hydroxyglycyrrhizinic acid, 18ẞ-glycyrrhizic acid, and 18α-glycyrrhizic acid are about 0.7, 1.0 and 1.2, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the peaks due to 18ẞ-glycyrrhizic acid and 18α-glycyrrhizic acid
Analysis
Samples: System suitability solution, Reference solution A, Reference solution B, and Sample solution
Acceptance criteria: See Table 1.
Table 1 | ||
| Name | Relative Retention Time | Acceptance Criteria |
| 24-Hydroxy glycyrrhizinic acida | 0.7 | NMT the sum of the areas of the peaks in the chromatogram from Reference solution B, corresponding to NMT 5.7% |
| Any other impurity | - | For each impurity, NMT 0.4 times the sum of the areas of the peaks in the chromatogram from Reference solution A, corresponding to NMT 2.0% |
| Sum of other impurities | - | NMT 1.6 times the sum of the areas of the peaks in the chromatogram from Reference solution A, corresponding to NMT 8.0% |
| Disregard limit | - | 0.04 times the sum of the areas of the peaks in the chromatogram from Reference solution A, corresponding to 0.2% |
a: (4β,20β)-3β-[[2-0-(B-D-Glucopyranosyluronic acid)-α-D-glucopyranosyluronic acid)oxy]-23-hydroxy-11-oxoolean-12-en-29-oic acid.
5 SPECIFIC TESTS
Change to read:
5.1 OPTICAL ROTATION, Specific Rotation (781S) (ERR-1-JUL-2022)
Sample solution: 10.0 mg/mL of Ammonium Glycyrrhizate in 50% ethanol
Acceptance criteria: +49.0° (ERR 1-Jul-2022) to +55.0" (ERR 1-Jul-2022) on the anhydrous basis
5.2 WATER DETERMINATION, Method la(921).
Sample: 0.25 g
5.3 Acceptance criteria: NMT 6.0%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight containers, and store in a cool, dry place.
6.2 USP REFERENCE STANDARDS (11)
USP Ammonium Glycyrrhizate RS
JSP Glycyrrhizic Acid RS.


