Amiodarone Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
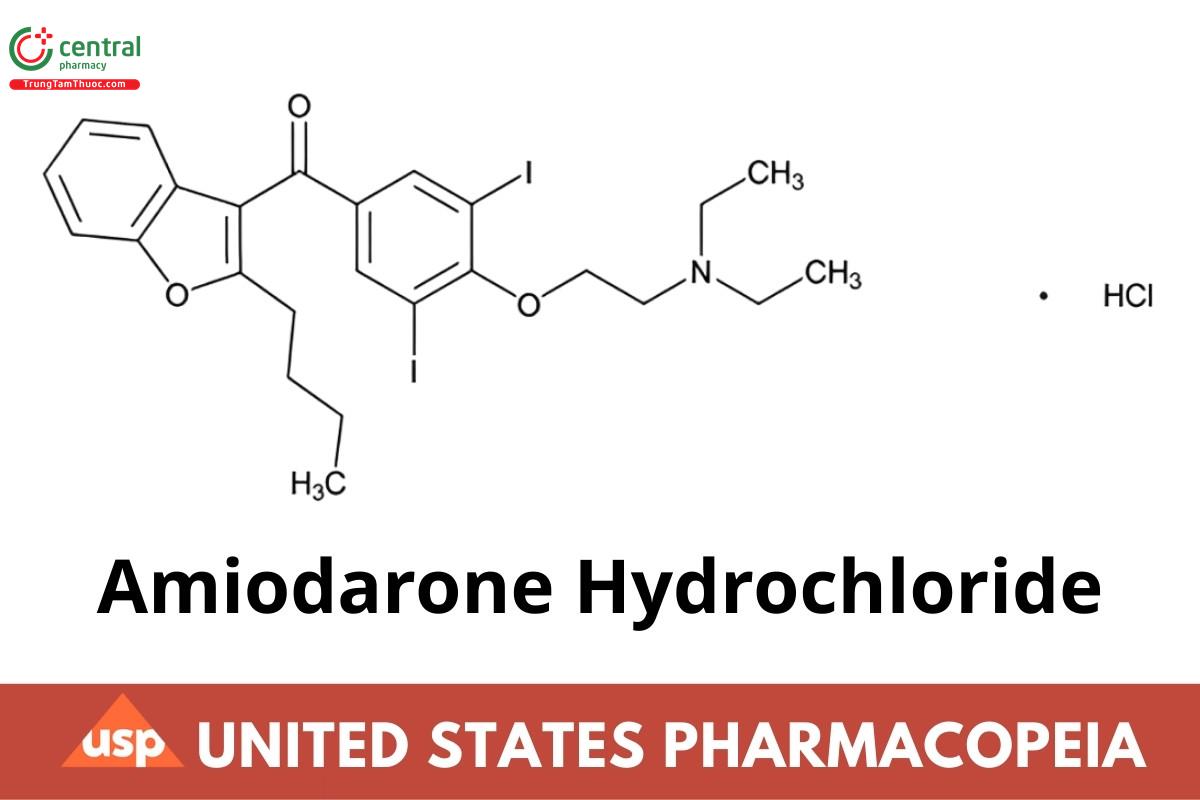
C25H29I2NO3·HCI 681.77
Methanone, (2-butyl-3-benzofuranyl) (4-[2-(diethyl amino)ethoxy]-3,5-diiodophenyl]-hydrochloride;
2-Butyl-3-benzofuranyl 4-[2-(diethylamino) ethoxy]-3,5-diiodophenyl ketone hydrochloride CAS RN®: 19774-82-4; UNII: 976728SY6Z.
2-Butyl-3-benzofuranyl 4-[2-(diethylamino) ethoxy]-3,5-diiodophenyl ketone CAS RN®: 1951-25-3; UNII: N3RQ532IUT.
1 DEFINITION
Amiodarone Hydrochloride contains NLT 98.5% and NMT 101.0% of C25H29I2NO3·HCI, calculated on the dried basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. IDENTIFICATION TESTS-GENERAL, Chloride (191): Meets the requirements
3 ASSAY
3.1 PROCEDURE
Buffer: Dissolve 6.80 g of monobasic potassium phosphate in 900 mL of water, and add 1.0 mL of triethylamine. Adjust with phosphoric acid to a pH of 6.00 ± 0.05, and dilute with water to 1000 mL.
Diluent: Acetonitrile and water (1:1)
Mobile phase: Acetonitrile and Buffer (1:1)
Standard stock solution: 0.5 mg/mL of USP Amiodarone Hydrochloride RS in methanol
Standard solution: 0.1 mg/mL USP Amiodarone Hydrochloride RS in Diluent from Standard stock solution
Sample stock solution: 0.5 mg/mL of Amiodarone Hydrochloride in methanol
Sample solution: 0.1 mg/mL of Amiodarone Hydrochloride in Diluent from Sample stock solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 3.9-mm x 15-cm; 5-µm packing L26
Flow rate: 1.5 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 1000 theoretical plates
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C25H29I2NO3·HCI in the portion of Amiodarone Hydrochloride taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of amiodarone in the Sample solution
rS = peak response of amiodarone in the Standard solution
CS = concentration of USP Amiodarone Hydrochloride RS in the Standard solution (mg/mL)
CU = nominal concentration of Amiodarone Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.5%-101.0%, on the dried basis
4 IMPURITIES
4.1 INORGANIC IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1% on a 1-g sample
4.2 ORGANIC IMPURITIES
[NOTE-The product meets the requirements for both Procedure 1 and Procedure 2.]
4.2.1 PROCEDURE 1
Potassium iodobismuthate solution: Dissolve 100 g of tartaric acid in 400 mL of water, and add 8.5 g of Bismuth subnitrate. Shake for 1 h, add 200 mL of a 400 g/L solution of potassium iodide, and shake well. Allow to stand for 24 h, filter, and protect from light.
Standard solution A: 0.02 mg/mL of USP Amiodarone Related Compound H. RS in methylene chloride
Standard solution B: Standard solution A and Sample solution (1:1).
Sample solution: 100 mg/mL of Amiodarone Hydrochloride in methylene chloride
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: Suitable layer of chromatographic silica gel and fluorescent indicator with maximum absorbance at 254 nm
Application volume
Standard solution A: 50 µL
Standard solution B: 100 µL
Sample solution: 50 µL
Developing solvent system: Methylene chloride, methanol, and anhydrous formic acid (17:2:1)
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Develop the plate in the Developing solvent system until the solvent front has moved NLT two-thirds the length of the plate, and dry in a current of cold air. Spray the plate with Potassium iodobismuthate solution and then with 3% Hydrogen peroxide solution. Examine immediately in daylight: the spot from Standard solution B due to amiodarone related compound H is clearly visible.
Acceptance criteria: Any spot with the same R, as the spot due to amiodarone related compound H from the Sample solution is not more intense than the spot from Standard solution A (0.02%).
4.2.2 PROCEDURE 2
Buffer: Add 3 mL of glacial acetic acid to 800 mL of water. Adjust with diluted ammonia solution to a pH of 4.9, and dilute with water to 1000 mL.
Mobile phase: Acetonitrile: methanol: Buffer (4:3:3 v/v/v).
Diluent: Acetonitrile and water (1:1)
Standard stock solution: Dissolve equal quantities of USP Amiodarone Related Compound D RS, USP Amiodarone Related Compound E RS, and USP Amiodarone Hydrochloride RS in a known amount of methanol.
Standard solution: 0.01 mg/mL each of USP Amiodarone Related Compound D RS, USP Amiodarone Related Compound E RS, and USP Amiodarone Hydrochloride RS, in Diluent from Standard stock solution
Sample solution: 5 mg/mL of Amiodarone Hydrochloride in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm x 15-cm; 5-µm packing L1
Column temperature: 30°
Flow rate: 1 mL/min
Injection size: 10 µL
Run time: 2 times the retention time of amiodarone
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 3.5 between amiodarone related compound D and amiodarone related compound E
Analysis
[NOTE-Disregard any peak that is less than 0.05%.]
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Amiodarone Hydrochloride taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of each impurity in the Sample solution
rS = peak response of amiodarone in the Standard solution
CS = concentration of USP Amiodarone Hydrochloride RS in the Standard solution (mg/mL)
CU = nominal concentration of Amiodarone Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria
Individual impurities: See Impurity Table 1.
Total impurities: NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Amiodarone related compound Aa | 0.26 | 0.2 |
| Amiodarone related compound Db | 0.29 | 0.2 |
| Amiodarone related compound Ec | 0.37 | 0.2 |
| Amiodarone related compound Bd | 0.49 | 0.2 |
| Amiodarone related compound Ce | 0.55 | 0.2 |
| Amiodarone related compound Gf | 0.62 | 0.2 |
| Amiodarone related compound Fg | 0.69 | 0.2 |
| Amiodarone hydrochloride | 1.00 | — |
| Any other individual impurity | — | 1.00 |
a (2-Butylbenzofuran-3-yl){4-[2-(diethylamino)ethoxy]phenyl}methanone.
b (2-Butylbenzofuran-3-yl)(4-hydroxy-3,5-diiodophenyl)methanone.
c (2-Butylbenzofuran-3-yl)(4-hydroxyphenyl)methanone.
d (2-Butylbenzofuran-3-yl){4-[2-(ethylamino)ethoxy]-3,5-diiodophenyl}methanone.
e (2-Butylbenzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3-iodophenyl}methanone.
f [2-[(1RS)-1-Methoxybutyl]benzofuran-3-yl][4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone.
g (2-Butylbenzofuran-3-yl)(4-hydroxy-3-iodophenyl)methanone.
5 SPECIFIC TESTS
5.1 LIMIT OF IODIDES
Solution A: Add 1.50 g of Amiodarone Hydrochloride to 40 mL of water at 80°, and shake until completely dissolved. Cool, and dilute with water to 50.0 mL.
Standard solution: To 15.0 mL of Solution A add 1.0 mL of 0.1 M hydrochloric acid, 1.0 mL of an 88.2 mg/L solution of potassium iodide, and 1.0 mL of 0.05 M potassium iodate. Dilute with water to 20.0 mL. Allow to stand protected from light for 4 h.
Sample solution: To 15.0 mL of Solution A add 1.0 mL of 0.1 M hydrochloric acid and 1.0 mL of 0.05 M potassium iodate. Dilute with water to 20.0 mL. Allow to stand protected from light for 4 h.
Analysis: Measure the absorbances of the Standard solution and the Sample solution at 420 nm, using a mixture of 15.0 mL of Solution A and 1.0 mL of 0.1 M hydrochloric acid diluted with water to 20.0 mL to serve as the blank. The absorbance of the Sample solution is NMT half the absorbance of the Standard solution.
Acceptance criteria: NMT 150 ppm
5.2 PH (791)
3.2-3.8. Dissolve 1 g of Amiodarone Hydrochloride in water by heating at 80°. Cool, and dilute with water to 20 mL..
5.3 LOSS ON DRYING (731)
Use 1 g of sample, and dry under vacuum (NMT 0.3 kPa) at 50° for 4 h: it loses NMT 0.5% of its weight.
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in light-resistant, tight containers. Store at controlled room temperature.
Change to read:
6.2 USP REFERENCE STANDARDS (11)
USP Amiodarone Hydrochloride RS
USP Amiodarone Related Compound D RS
(2-Butylbenzofuran-3-yl) (4-hydroxy-3,5-diiodophenyl) methanone.
C19H16I2O3 546.14
USP Amiodarone Related Compound E RS
(2-Butylbenzofuran-3-yl) (4-hydroxyphenyl) methanone.
C19H18O3 294.34
USP Amiodarone Related Compound H RS
2-Chloro-N,N-diethylethanamine hydrochloride (ERR 1-Dec-2020).
C6H14CIN·HCI 172.09 (ERR 1-Dec-2020)


