Aminosalicylic Acid
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
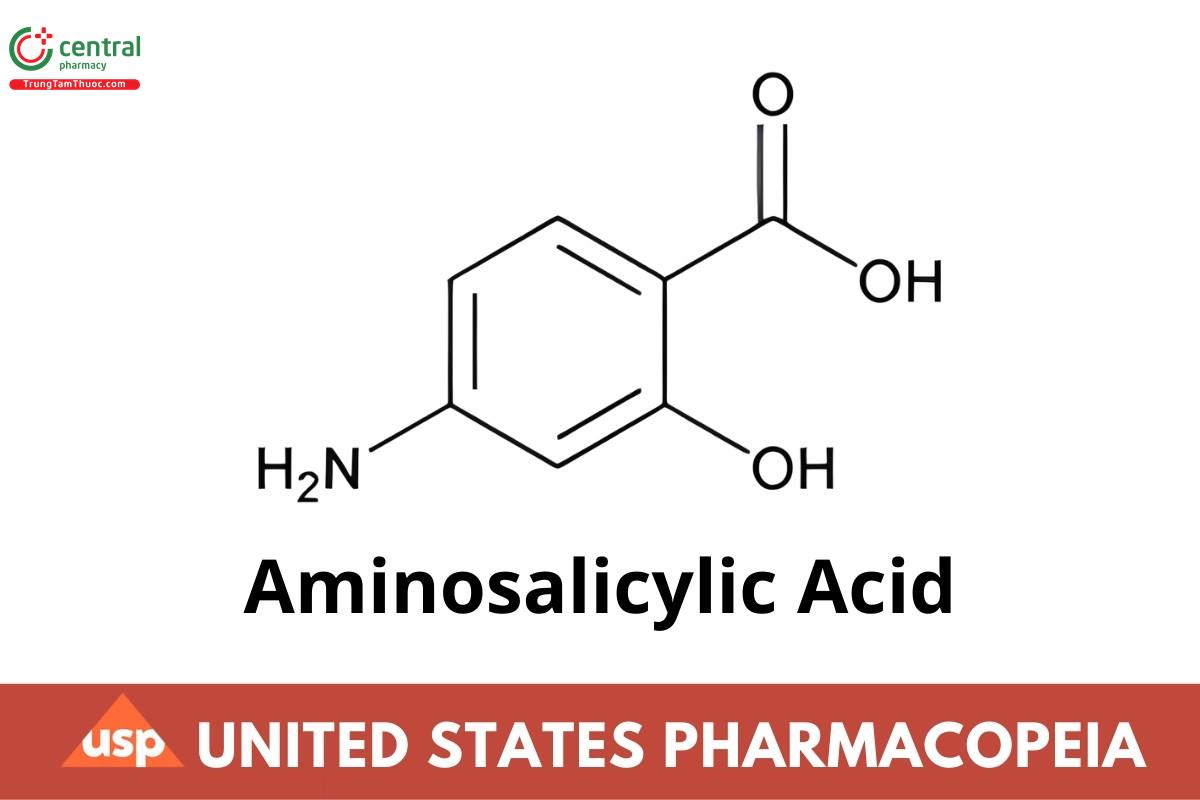
C7H7NO3 153.14
Benzoic acid, 4-amino-2-hydroxy-;
4-Aminosalicylic acid CAS RN®: 65-49-6; UNII: 582658E0N2.
1 DEFINITION
Aminosalicylic Acid contains NLT 98.5% and NMT 100.5% of aminosalicylic acid (C7H7NO3), calculated on the anhydrous basis.
[CAUTION-Under no circumstances use a solution prepared from Aminosalicylic Acid if its color is darker than that of a freshly prepared solution.]
2 IDENTIFICATION
2.1 A.
Sample stock solution: Dissolve 250 mg in 3 mL of 1 N sodium hydroxide, transfer to a 500-mL volumetric flask, and dilute with water to volume.
Sample solution: Transfer a 5-mL aliquot of the Sample stock solution to a 250-mL volumetric flask containing 12.5 mL of pH 7 phosphate buffer (see Reagents, Indicators, and Solutions-Buffer Solutions), and dilute with water to volume.
Analysis: Compare the Sample solution in a suitable spectrometer against a blank of the same buffer in the same concentration.
Acceptance criteria: The Sample solution exhibits absorbance maxima at 265 ± 2 and 299 ± 2 nm, and the ratio A265/A299 is 1.50-1.56.
2.2 B.
Sample: 1 g
Analysis: Place the Sample in a small, round-bottom flask, and add 10 mL of acetic anhydride. Heat the flask on a steam bath for 30 min, add 40 mL of water, filter, cool, and allow to stand until the diacetyl derivative has crystallized. Collect the precipitate on a filter, wash well with water, and dry at 105° for 1 h.
Acceptance criteria: The diacetyl derivative melts at 191°-197°.
2.3 C.
Sample: 0.1 g
Analysis: Shake the Sample with 10 mL of water, and filter. To 5 mL of the filtrate add 1 drop of ferric chloride TS.
Acceptance criteria: A violet color is produced.
3 ASSAY
3.1 PROCEDURE
Solution A: 12.7 mg/mL of tetrabutylammonium hydroxide in methanol
Mobile phase: Solution A, 0.05 M dibasic sodium phosphate, and 0.05 M monobasic sodium phosphate (150:425:425)
Internal standard solution: 5 mg/mL of Acetaminophen in Mobile phase
Standard solution: 0.5 mg/mL of aminosalicylic acid prepared as follows. Transfer 12.5 mg of USP Aminosalicylic Acid RS to a 25-mL low-actinic volumetric flask, add 15 mL of Mobile phase, and swirl to dissolve. Add 2.5 mL of the Internal standard solution, and dilute with Mobile phase to volume.
Sample solution: 0.5 mg/mL of Aminosalicylic Acid prepared as follows. Transfer 12.5 mg of Aminosalicylic Acid to a 25-mL low-actinic volumetric flask, add 15 mL of Mobile phase, and swirl to dissolve. Add 2.5 mL of the Internal standard solution, and dilute with Mobile phase to volume.
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
3.3 System suitability
Sample: Standard solution
[NOTE-The relative retention times for acetaminophen and aminosalicylic acid are 0.83 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.7 between aminosalicylic acid and acetaminophen
Relative standard deviation: NMT 1.0% for the peak response ratio of aminosalicylic acid to acetaminophen
3.4 Analysis
Samples: Standard solution and Sample solution
After use, wash the column for 30 min with a mixture of methanol, water, and phosphoric acid (77:23:0.6), and then wash for 30 min with a mixture of methanol and water (50:50).
Calculate the percentage of aminosalicylic acid (C7H7NO3) in the portion of Aminosalicylic Acid taken:
Result = (RU/RS) x (CS/CU) x 100
RU = peak response ratio of aminosalicylic acid to acetaminophen from the Sample solution
RS = peak response ratio of aminosalicylic acid to acetaminophen from the Standard solution
CS = concentration of USP Aminosalicylic Acid RS in the Standard solution (mg/mL)
CU = concentration of Aminosalicylic Acid in the Sample solution (mg/mL)
Acceptance criteria: 98.5%-100.5% on the anhydrous basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.2%
4.2 CHLORIDE AND SULFATE, Chloride(221)
Sample solution: 25 mg/mL in a mixture of nitric acid and water (5:15)
Acceptance criteria: NMT 0.042%; the solution shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid.
4.3 LIMIT OF M-AMINOPHENOL
Solution A: 12.7 mg/mL of tetrabutylammonium hydroxide in methanol
Mobile phase: Solution A, 0.05 M dibasic sodium phosphate, and 0.05 M monobasic sodium phosphate (150:425:425)
Internal standard solution: 5 µg/mL of sulfanilamide in Mobile phase
Standard stock solution: 12 µg/mL of USP m-Aminophenol RS in Mobile phase
Standard solution: 1.2 µg/mL of USP m-Aminophenol RS prepared as follows. Transfer 10.0 mL of the Standard stock solution and 10.0 mL of the Internal standard solution to a 100-mL low-actinic volumetic flask, and dilute with Mobile phase to volume.
Sample solution: 0.5 mg/mL of Aminosalicylic Acid prepared as follows. Transfer 50 mg of Aminosalicylic Acid to a 100-mL low-actinic volumetic flask, add 50 mL of Mobile phase, and swirl to dissolve. Add 10.0 mL of the Internal standard solution, and dilute with Mobile phase to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 25-cm; 10-µm packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
[NOTE-The relative retention times for sulfanilamide and m-aminophenol are about 0.66 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.5 between m-aminophenol and sulfanilamide
Relative standard deviation: NMT 7%
Analysis
Samples: Standard solution and Sample solution
After use, wash the column for 30 min with a mixture of methanol, water, and phosphoric acid (77:23:0.6), and then wash for 30 min with a mixture of methanol and water (50:50).
Calculate the percentage of m-aminophenol in the portion of Aminosalicylic Acid taken:
Result = (RU/RS) x (CS/CU) x 100
RU = peak response ratio of m-aminophenol to sulfanilamide from the Sample solution
RS = peak response ratio of m-aminophenol to sulfanilamide from the Standard solution
CS = concentration of USP m-Aminophenol RS in the Standard solution (mg/mL)
CU = concentration of the Sample solution, as determined in the Assay (mg/mL)
Acceptance criteria: NMT 0.25%
5 SPECIFIC TESTS
5.1 PH (791)
3.0-3.7, in a saturated solution
5.2 WATER DETERMINATION, Method (921)
NMT 0.5%
5.3 HYDROGEN SULFIDE, SULFUR DIOXIDE, AND AMYL ALCOHOL
Sample: 500 mg
Analysis: Dissolve the Sample in 5 mL of 1 N sodium hydroxide, add 6 mL of 3 N hydrochloric acid, and stir vigorously.
Acceptance criteria: No odor of hydrogen sulfide or sulfur dioxide is perceptible, and NMT a faint odor of amyl alcohol is perceptible. A piece of moistened lead acetate test paper held over the mixture does not become discolored.
5.4 CLARITY AND COLOR OF SOLUTION
Sample 1: 1 g
Analysis 1: Dissolve Sample 1 in 10 mL of sodium bicarbonate solution (1 in 15).
Acceptance criteria 1: The resulting solution is clear and has NMT a faint yellow color.
Sample 2: 1 g
Analysis 2: Dissolve Sample 2 in 50 mL of freshly prepared 1.6 M nitric acid.
Acceptance criteria 2: The resulting solution is clear and has NMT a slight color.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers at a temperature not exceeding 30°.
USP REFERENCE STANDARDS (11).
USP m-Aminophenol RS
USP Aminosalicylic Acid RS


