Aminohippuric Acid
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
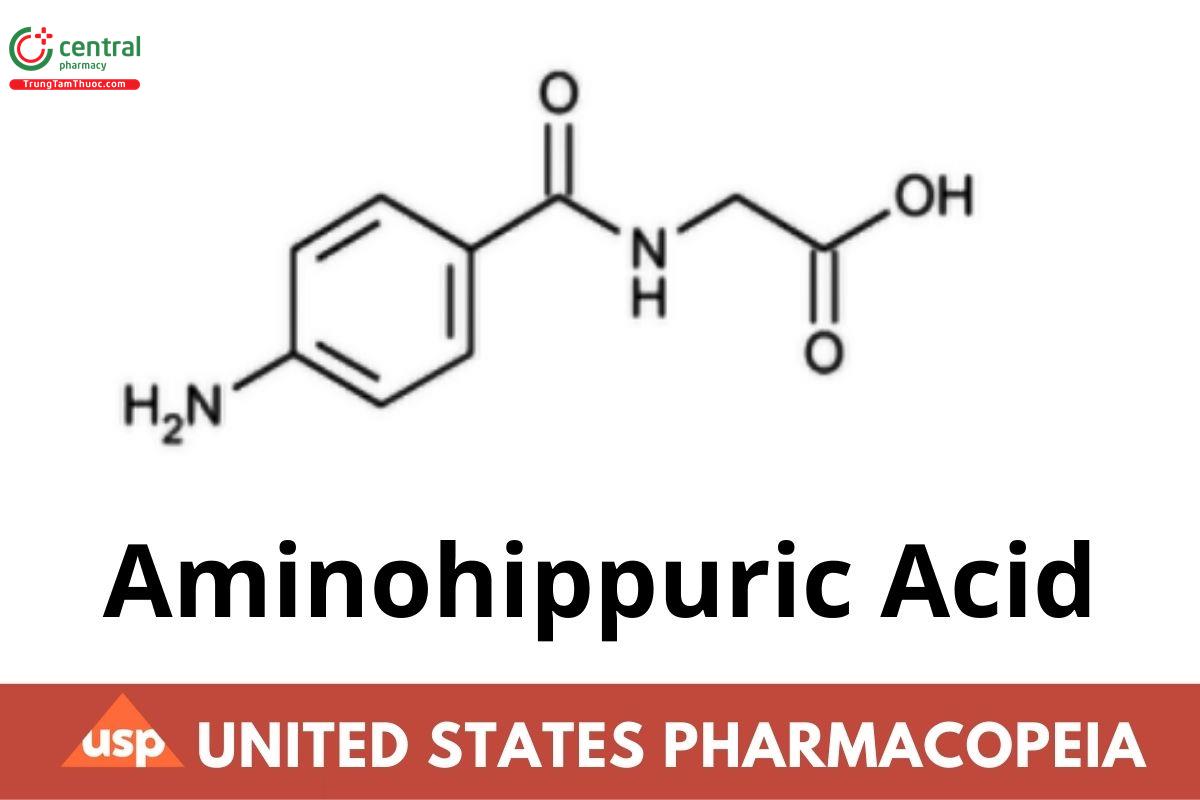
C9H10N2O3 194.19
Glycine, N-(4-aminobenzoyl)-;
p-Aminohippuric acid CAS RN®: 61-78-9; UNII: Y79XT83BJ9.
1 DEFINITION
Aminohippuric Acid contains NLT 98.0% and NMT 100.5% of aminohippuric acid (C9H10N2O3 ), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B.
Sample: 10 mg of Aminohippuric Acid
Analysis: Dissolve the Sample in 5 mL of water, add 0.5 mL of 3 N hydrochloric acid, 0.5 mL of sodium nitrite solution (1 in 10), and a solution of 0.20 g of 2-naphthol in 10 mL of 6 N ammonium hydroxide.
Acceptance criteria: A red color is produced.
3 ASSAY
PROCEDURE
Sample: 150 mg of Aminohippuric Acid
Analysis: To the Sample add 5 mL of hydrochloric acid and 50 mL of water. Proceed as directed in Nitrite Titration (451), beginning with "stir until dissolved." Each mL of 0.1 M sodium nitrite is equivalent to 19.42 mg of aminohippuric acid (C9H10N2O3 ).
Acceptance criteria: 98.0%-100.5% on the dried basis
4 IMPURITIES
RESIDUE ON IGNITION (281 ): NMT 0.25%
5 SPECIFIC TESTS
LOSS ON DRYING (731).
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 0.25%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
USP REFERENCE STANDARDS (11)
USP Aminohirpuric Acid RS.


